Consider a mixture of species 1, 2, and 3. The following equation of state is available for
Question:
Consider a mixture of species 1, 2, and 3. The following equation of state is available for the vapor phase: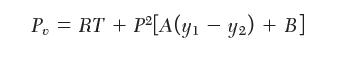
where,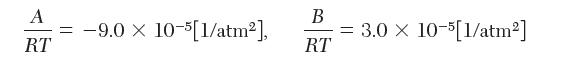
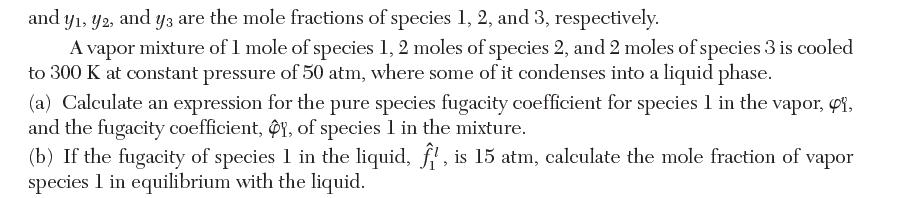
Transcribed Image Text:
P = RT + P [A(y - y) + B]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Consider a mixture of species 1 and 2 in vaporliquid equilibrium at 25C and 90 bar. The following equation of state is available for the vapor phase: and y1 and y2 are the mole fractions of species 1...
-
Consider a mixture of species 1, 2, and 3. The following equation of state is available for the vapor phase: where, and y1, y2, and y3 are the mole fractions of species 1, 2 and 3, respectively....
-
A vaporliquid phase diagram for a binary mixture of species 1 and 2 at 293 K is shown in the following fi gure. Answer the following questions. (a) At 293 K, what is the value of P 1 sat ? (b)...
-
Migration is a popular strategy among many species. Monarch butterflies migrate between the Sierra Madre mountains in Mexico and many locations across the USA and Canada. Answer the following...
-
1- Briefly describe the background that led to the FBI initiation of the virtual case file (VCF) project. What do you think were the reasons why the VCF project failed? How do you relate your...
-
Little Oil has outstanding 1 million shares with a total market value of $20 million. The firm is expected to pay $1 million of dividends next year, and thereafter the amount paid out is expected to...
-
A Behavioral Theory of the Firm (BTF; Cyert & March, 1963) is one of the most significant management books of all time. Why is this the case? Explain and describe a specific influence the theory has...
-
The following pertains to the Cereal Division of McKenzie Corporation. Conversion costs for this division were 80 percent complete as to beginning work-in-process inventory and 50 percent complete as...
-
The comparative balance sheet of Navaria Inc. for December 31, 20Y3 and 20Y2, is as follows: Dec. 31, 20Y3 Dec. 31, 20Y2 Assets Cash $ 202,870 $ 191,470 Accounts receivable (net) 74,410 68,270...
-
A mixture of 2 moles propane (1), 3 moles butane (2), and 5 moles pentane (3) is contained at 30 bar and 200C. The van der Waals constants for these species are: Determine the fugacity and fugacity...
-
(a) Calculate the fugacity of pure methane vapor at T = 190.6 K and P = 32.2 bar. (b) Using the Lewis fugacity rule, calculate the fugacity of methane in a mixture of 80 mol% methane and 20 mol%...
-
Unions can affect (a) A firms profits, (b) The price consumers pay for a good, and (c) The wages received by non-union workers. Do you agree or disagree? Explain your answer.
-
In countries with high unemployment and poverty rates, the nation's people are often more concerned with the economic environment than the intricacies of its political systems. In 2010, Mohamed...
-
Analyze this approach: Consider that you could increase the productivity of your department, you have thought about certain ways to do it, but you are not sure. Your team has a lot of experience, but...
-
What is the research question or objective? What research methods did the authors use? Examples include survey, case study, interviews, opinions, qualitative, quantitative, etc. What are the...
-
Provide a critical reflection on each department outlining which services, aspects and operational factors you should further investigate to help improve customer satisfaction. Express the negative...
-
1. Many courses use group projects. What are some of the things that make positive group project experiences? 2. How can a manager motivate employees? Give some specific ideas. Include when you've...
-
Explain the concept of positioning. How is a companys positioning likely to be related to the strategic prominence of price in that company?
-
Assume today is the 21st of February. Using the information below, FT Extract, answer the following questions (parts i and ii). You work for a US company that is due to receive 250 million in June...
-
Acetic acid was evaporated in container of volume 21.45 cm3 at 437 K and at an external pressure of 101.9 kPa, and the container was then sealed. The mass of acid present in the sealed container was...
-
The dissociation of I, can be monitored by measuring the total pressure, and three sets of results are as follows: T/K 973 1073 1173 100p/atm 6.244 7.500 9.181 104nj 2.4709 2.4555 2.4366 Where n1 is...
-
The 1980s saw reports of fH (SiH2) ranging from 243 to 289 k] mol-1. For example, the lower value was cited in the review article by R. Walsh (Ace. Chem. Res. 14,246 (1981)); Walsh later leant...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


