Consider a system initially charged with 1 mole of pure I2 that is maintained at 1300 K
Question:
Consider a system initially charged with 1 mole of pure I2 that is maintained at 1300 K and 1 bar in which the following dissociation reaction occurs: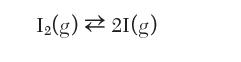
For monatomic iodine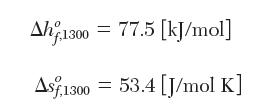
Plot ΔH, TΔS and ΔG as a function of extent of reaction. What is the equilibrium conversion?
Transcribed Image Text:
1(g) 21(g)
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Solve the multiple chemical reaction equilibrium problem in Example 9.19 at 800 K using the following set of independent reactions: Example 9.19 Consider a system initially charged with 1 mole of...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
(a) LEP Table 12-2: Exothermic Reaction with Heat Exchange Download the Polymath, MATLAB, Python, or Wolfram codes for the algorithm and data given in Table T12-2 for the exothermic gas phase...
-
Suppose we have an automatic module on the module path named lizard-^-cricket-^-1.0.0-SNAPSHOT.jar and no Automatic-Module-Name specified. What module name should named modules use to reference it?...
-
You are faced with two investments. One is very risky, but the potential returns are high. The other is safe, but the potential is quite limited. Pick one.
-
At t = 0, a massless bucket contains a mass M of sand. It is connected to a wall by a massless spring with constant tension T (that is, independent of length).18 See Fig. The ground is frictionless,...
-
Refer to Exercise 11.107. a. Using no information about x, estimate and calculate a 95% confidence interval for the mean value of y. [Hint: Use the one-sample t methodology of Section 7.3.] b. Plot...
-
Suppose your friend is thinking of opening a new' restaurant, and she hopes that she will have around 20 groups of (on average) 4 customers on a typical busy evening. Each meal will take around 1.5...
-
Bennett Retallers had the following transactions in November and December: Required: Compute net sales for the two months ended December 31. Note: Do not round your intermedlate calculations. Round...
-
Calculate the Gibbs energy of formation of NH3 at 1000 K. Remember that the Gibbs energies of formation of the elements are still zero at this temperature.
-
At 25C and 1 atm, the Gibbs energy of reaction to produce liquid hydrogen peroxide (H2O2) from liquid water has been measured to be 116.8 kJ/mol. From this value determine the (Agf. 298) HO*
-
Refer to the Archives of Pediatrics and Adolescent Medicine (Dec. 2007) study of honey as a children's cough remedy, In addition to the two experimental groups of children with an upper respiratory...
-
What should be the equivalent units of production for (1) Dept M and (2) Dept. P? Can you please show the solutions and answer. Thanks Problem 1 Lee Gon Mfg. Co has its product processed in two...
-
Moullierat Mfg. is considering a rights offer. The company has determined that the ex-rights price will be $95. The current price is $102 per share, and there are 24 million shares outstanding. The...
-
This question involves hypothesis testing. The following numbers will help you answer these questions. The random variable Z ~N(0, 1) is standard normal. P(Z >1.28).1 P(Z1.65) .05 P(Z1.96) .025 P(Z...
-
Human service organizations require strong and effective leadership. Understanding what qualities make up an effective leader and how these qualities can be cultivated is of critical importance for...
-
18. What is the name of the heat treatment performed on a cold worked sample? 19. What is the percent coldwork of a sample with an initial thickness of 11mm and a final thickness of 7mm? 20. Which...
-
Refer to Problem 58. Determine the amount of taxable income and separately stated items in each case, assuming the corporation was a Subchapter S corporation. Ignore any carry forward items. a....
-
Provide an example of an aggressive accounting practice. Why is this practice aggressive?
-
Ammonium cyanate is composed of an ammonium caution (NH 4 + ) and a cyanate anion (OCN ). Show a Lewis structure for the cyanate anion. (Both O and N are bonded to C.) Which atom has the negative...
-
(a) Show a Lewis structure for urea. CH 4 N 2 O. Both Ns and the O are bonded to the C. The Hs are bonded to the Ns. None of the atoms has a formal charge. (b) Show a Lewis structure of an isomer of...
-
Phosphorus forms two compounds with chlorine, PCl 3 , and PCl 5 . The former follows the octet rule, but the latter does not. Show Lewis structures for each of these compounds. For the corresponding...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


