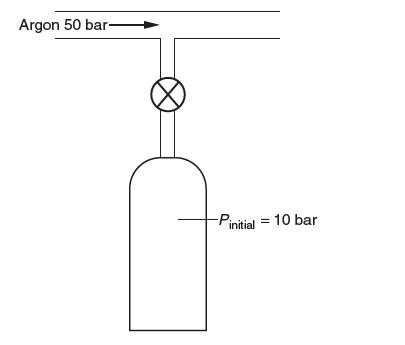
Consider fi lling a cylinder of compressed argon from a high-pressure supply line as shown below. Before
Question:
Consider fi lling a cylinder of compressed argon from a high-pressure supply line as shown below. Before fi lling, the cylinder contains 10 bar of argon at room temperature. The valve is then opened, exposing the tank to a 50 bar line at room temperature until the pressure of the cylinder reaches 50 bar. The valve is then closed. For argon take cP = (5/2)R and the molecular weight to be 40 kg/kmol. You may use the ideal gas model.
(a) What is the temperature right after the valve is closed?
(b) If the cylinder sits in storage for a long time, how much heat is transferred (in kJ/kg)?
(c) What is the pressure of the cylinder when it is shipped (after it was stored for a long time)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





