Consider the production of 1,1-dichloroethane (C2H4Cl2) from ethylene (C2H4) and chlorine (Cl2). This gas-phase reaction is the
Question:
Consider the production of 1,1-dichloroethane (C2H4Cl2) from ethylene (C2H4) and chlorine (Cl2). This gas-phase reaction is the fi rst step in producing polyvinyl chloride (PVC). The feed ratio of reactants is 2 moles chlorine: 1 mole ethylene. You may assume Dhrxn o does not change with temperature.
(a) Calculate the maximum temperature at which 90.0% conversion can be obtained at a pressure of 1 bar.
(b) Consider an increase in pressure to 30 bar. What is the conversion obtained at the same temperature as that calculated in part (a)? You may assume that the Lewis fugacity rule applies and use the virial truncated form of the van der Waals equation. The following van der Waals constants are available: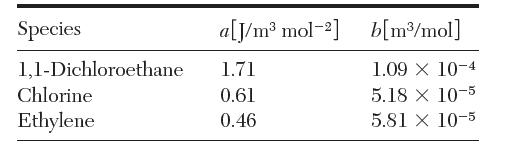
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





