Consider the production of hydrogen by the water gas shift reaction: The feed contains an equal amount
Question:
Consider the production of hydrogen by the water gas shift reaction: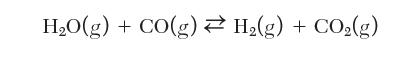
The feed contains an equal amount of CO and H2. Water is present at 500% excess of its stoichiometric requirement. Calculate the equilibrium composition at 1000 K and 1 bar.
Transcribed Image Text:
HO(g) + CO(g) H(g) + CO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Joan Gakii
I'm a meticulous professional writer with over five years writing experience. My skill set includes
- Digital Content,
- Interpersonal Communication,
- Web Content and academic Writing,
- Proofreading,
- Editing,
- Project Management, and
- Public Relations.
5.00+
7+ Reviews
12+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
The autothermal reforming of methane to hydrogen was described in Example 4.2. The solution in the example was not optimized, and suggestions were given for how to improve the results. Optimize the...
-
Consider the production of a synthetic fuel (methanol) from coal. A gas mixture of 50% CO and 50% H2 leaves a coal gasifier at 500 K, 1 MPa, and enters a catalytic converter. A gas mixture of...
-
People who earn a higher salary can afford more goods, including health care. However, according to Grossman, they will choose a higher desired health stock. Why is this so, according to the model?
-
1- Summarize the relationships among labor, land, and capital as they relate to production. 2- What do you think are the most influential factors that contribute to earnings? 3- Research income...
-
A block and tackle is used to support a mass of 120 kg as shown in Figure. (a) What is the tension in the rope? (b) What is the mechanical advantage of this device? 120ka
-
RateMyProfessors.com. A popular Web site among college students is RateMyProfessors.com (RMP). Established over 10 years ago, RMP allows students to post quantitative ratings of their instructors. In...
-
Can Idle v. City Co. be authority at all? Why or why not?
-
Task 2: Determine your portfolio's capital allocation/best weights, as well as its risk and return. Lo2, LO3 & L04 (Marks: 25) Once your portfolio is complete, use the following formula to calculate...
-
Consider the molecular dissociation of diatomic oxygen to monatomic oxygen What is the minimum temperature required to get 10% O at 1 bar? How can you change the pressure to lower the minimum...
-
You have obtained the following equilibrium data for the reaction: with a stoichiometric feed of A and B. At 200C and 1 bar, 25% of the species in the reactor were the product C. At 300C and 1 bar,...
-
The books Web site (wvvw.stat.ufl.edu/ ¼aa/cda/cda.html) has a 3 Ã 4 Ã 4 table that cross-classifies dumping severity (Y) and operation (X) for four hospitals (H). The four...
-
Question 1 [40 marks] (a) Table 1 present experimental data related to the absorbance of two compounds over a range of concentration, in a UV-Vis cell with path length I = 1.0 cm. From this table:...
-
i. The following table presents data on wholesale gas prices for the major capital cities in the Eastern-half of Australia, from 2011-12 to 2022-23. Use this data to construct a single, time-series...
-
Problem 1 Using the same Fourier-Method approach as used in lecture, consider a beam loaded as shown below. 290 -q. Cos 280 x Shane land V-280 Distributed load w = =-80 . Cos[X] a. What are the...
-
Think about a Floor Warden training program for that company - and write me another email (attached here as a Word document) as if I were the leader of your organization to tell me about the...
-
A particle travels around the curve shown, following ? = ? 0 . 2 ? ? , ?with ? ( ? ) = 0 . 5 ? 2 rad. At the moment ? = ? , ?determine the speed and acceleration of the particle. ? = , ? ? ? = , ? ?...
-
A homeowner has asked a local real estate agent for advice on the price he should set for his house. The real estate agent notes that the only comparable house in the neighborhood that is currently...
-
When the concentration of a strong acid is not substantially higher than 1.0 10-7 M, the ionization of water must be taken into account in the calculation of the solution's pH. (a) Derive an...
-
For how long on average would an atom remain on a surface at 400 K if its desertion activation energy were? (a) 20 kO mol-1, (b) 200 k] mol-I? Take TO = 0.12 ps. For how long on average would the...
-
A solid in contact with a gas at 8.86 kPa and 25C adsorbs 4.67 mg of the gas and obeys the Langmuir isotherm. The enthalpy change when 1.00 mmol of the adsorbed gas is desorbed is +12.2 J. What is...
-
Suppose it is known that ozone adsorbs on a particular surface in accord with a Langmuir isotherm. How could you use the pressure dependence of the fractional coverage to distinguish between...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


