For a given binary system at constant T and P, the molar volume (in cm3 /mol) is
Question:
For a given binary system at constant T and P, the molar volume (in cm3 /mol) is given by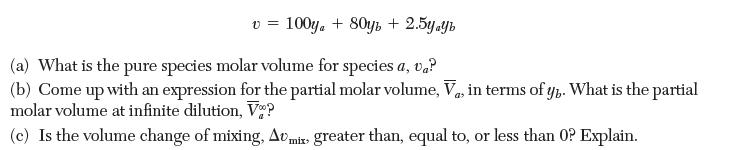
Transcribed Image Text:
v = 100y + 80y + 2.5yayb (a) What is the pure species molar volume for species a, va? (b) Come up with an expression for the partial molar volume, Va, in terms of y. What is the partial molar volume at infinite dilution, V? (c) Is the volume change of mixing, Amix greater than, equal to, or less than 0? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)

Answered By

FREDRICK MUSYOKI
Professional Qualities:
Solution-oriented.
Self-motivated.
Excellent problem-solving and critical thinking skills.
Good organization, time management and prioritization.
Efficient troubleshooting abilities.
Tutoring Qualities:
I appreciate students as individuals.
I am used to tailoring resources for individual needs.
I can integrate IT into student's lessons.
I am good at explaining concepts.
I am able to help students progress.
I have a wide curriculum knowledge.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Department K had 3,000 units 45% completed in process at the beginning of the period; 17,000 units completed during the period; and 1,200 units 40% completed at the end of the period. What was the...
-
In some cases if pure liquid A and pure liquid B are mixed at constant temperature and pressure, two liquid phases are formed at equilibrium, one rich in species A and the other in species B. We have...
-
Correction, overproduction, inventory and motion are all examples of: a. Waste b. 5 S target areas c. Noise d. Value-added activities
-
You have just won the Strayer Lottery jackpot of $11,000,000. You will be paid in 26 equal annual installments beginning immediately. If you had the money now, you could invest it in an account with...
-
Sigma Corporation applies overhead cost to jobs on the basis of direct labor cost. Job V, which was started and completed during the current period, shows charges of $5,000 for direct materials,...
-
PROBLEM 221 Plantwide Versus Multiple Predetermined Overhead Rates LO21, LO22 Mason Company has two manufacturing departmentsMachining and Assembly. The company considers all of its manufacturing...
-
Adden Company signs a lease agreement dated January 1, 2019, that provides for it to lease non-specialized heavy equipment from Scott Rental Company beginning January 1, 2019. The lease terms,...
-
Windhoek Mines, Limited, of Namibia, is contemplating the purchase of equipment to exploit a mineral deposit on land to which the company has mineral rights. An engineering and cost analysis has been...
-
Consider a mixture of species 1, 2, and 3. The following equation of state is available for the vapor phase: where, and y1, y2, and y3 are the mole fractions of species 1, 2 and 3, respectively....
-
Consider an ideal gas mixture at 83.14 kPa and 500 K. It contains 2 moles of species A and 3 moles of species B. Calculate the following:VA, VB, vA, vB, VA, VB, V, v, Vmix, vmix.
-
Find the lift on an airfoil using Figure 4.12 and Table 2.1 given the following information: AOA = 12 Wing area, S = 110 ft2 TAS = 130 kts. Pressure altitude = 8000 ft Temperature Standard Day Level...
-
1. A T-shaped beam with an overhang is supported and loaded as shown in Fig. 1. Draw shear force diagram and calculate (a) the shear stress at a point D, 2 m from support A and 25 mm from the top of...
-
Question 1: Write Specific Case to brief is Flying Fish Bikes, Inc. v. Giant Bicycle, Inc., 181 F.Supp.3d 957? Grading Rubric for Case Brief Written Case Brief 3 (Exceeds Identification - Heading...
-
A local gym is looking in to purchasing more exercise equipment and runs a survey to find out the preference in exercise equipment amongst their members. They categorize the members based on how...
-
A fountain with an opening of radius 0.015 m shoots a stream of water vertically from ground level at 6.0 m/s. The density of water is 1000 kg/m. (a) Calculate the volume rate of flow of water. (b)...
-
35 h/2 21 3 3 2t 3. A thin-walled beam has the cross-section shown in the figure. If the beam is subjected to a bending moment Mx in the plane of the web 23 calculate the direct stress at the points...
-
The managers of a washing machine manufacturer are considering introducing a new super-premium model to the companys product line. They recognize that the high price of this new model will cause its...
-
A glass manufacturer produces hand mirrors. Each mirror is supposed to meet company standards for such things as glass thickness, ability to reflect, size of handle, quality of glass, color of...
-
1, 3, 5-hexatriene (a kind of 'linear' benzene) was converted into benzene itself. On the basis of a free-electron molecular orbital model (in which hexatriene is treated as a linear box and benzene...
-
The following data were obtained for the absorption by a dye dissolved in methylbenzene using a 2.50 mm cell. Calculate the molar absorption coefficient of the dye at the wavelength employed:...
-
A 2.50-mm cell was filled with a solution of a dye. The concentration of the dye was 15.5 mmol dm-3 Calculate the molar absorption coefficient of benzene at this wavelength given that the...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


