One mole per second of gas at 10 bar and 500C fl ows through an isentropic, adiabatic
Question:
One mole per second of gas at 10 bar and 500°C fl ows through an isentropic, adiabatic turbine, where it exits at 1 bar. This gas can be described by the equation of state,![]()
Where b = 5 * 1024 m3/mol. The ideal gas heat capacity is given by cv = 3/2 R. Answer the following questions:
(a) What is the exit temperature?
(b) Is the coeffi cient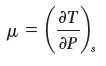
positive, zero, or negative for this process? Is this consistent with your result in part A?
(c) What is the work obtained by the turbine, in [J/mol]?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





