Solid titanium can be produced by reacting titanium tetrachloride gas with molten magnesium liquid using the following
Question:
Solid titanium can be produced by reacting titanium tetrachloride gas with molten magnesium liquid using the following reaction:
![]()
You wish to react 40% of the magnesium liquid at constant pressure and a temperature of 1000 K.
Gibbs energy of formation data at 1000 K are as follows: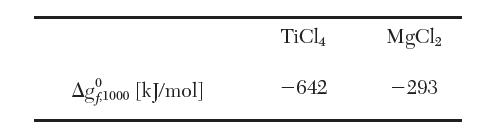
(a) For every kg of magnesium placed in the reactor, how many kg of Ti will be produced?
(b) Calculate the approximate pressure needed. You can assume ideal gas and ideal liquid behavior.
(c) Explain how you could calculate the pressure more accurately than in part (a). What data would you need?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





