The excess Gibbs energy for a binary mixture of liquid a and liquid b is given by:
Question:
The excess Gibbs energy for a binary mixture of liquid a and liquid b is given by:![]()
where T is in [K]. The solids of these species are completely immiscible. The enthalpies of fusion and melting temperatures are as follows: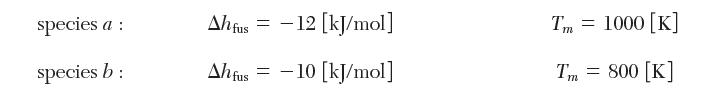
Determine the temperature and the composition at the eutectic point. You may neglect the change in heat capacity between the solid and liquid phases.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





