The following data are reported for a binary mixture of species 1 and 2 at 40C. The
Question:
The following data are reported for a binary mixture of species 1 and 2 at 40°C. The activity coeffi cients at infi nite dilution are: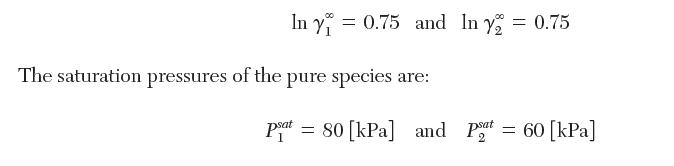
Answer the following questions:
(a) 4 moles of species 1 and 6 moles of species 2 are placed in a closed container and allowed to come to vapor–liquid equilibrium at 40°C. The liquid mole fraction is measured to be x1 = 0.32.
What is the pressure in the system? What is the gas phase mole fraction? How many total moles of liquid are there in the system?
(b) Does this system form an azeotrope at 40°C? If so, at what pressure and composition? If not, explain.
(c) Are the “like” interactions stronger than the “unlike”? Explain.
(d) Estimate the enthalpy of mixing. State any assumptions that you make.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





