The following expression describes the relation between pressure and molar volume of pure SF6 vapor at 30C,
Question:
The following expression describes the relation between pressure and molar volume of pure
SF6 vapor at 30°C, where P is in bar and v is in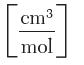
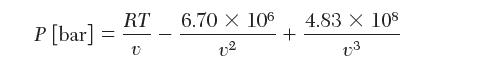
Consider a container that is 10 L (10,000 cm3) that contains 10.79 mol of SF6 vapor at 30°C.
Answer the following.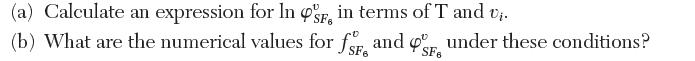
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





