The process in Example 5.2 indicates that we need to put work into the system during an
Question:
The process in Example 5.2 indicates that we need to put work into the system during an expansion process. Determine whether this result is possible (in a thermodynamic sense); if it is, explain this result physically.
EXAMPLE 5.2
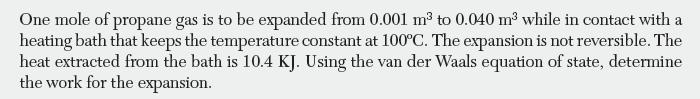
Transcribed Image Text:
One mole of propane gas is to be expanded from 0.001 m to 0.040 m while in contact with a heating bath that keeps the temperature constant at 100C. The expansion is not reversible. The heat extracted from the bath is 10.4 KJ. Using the van der Waals equation of state, determine the work for the expansion.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Let A, B be sets. Define: (a) the Cartesian product (A B) (b) the set of relations R between A and B (c) the identity relation A on the set A [3 marks] Suppose S, T are relations between A and B, and...
-
can someone solve this Modern workstations typically have memory systems that incorporate two or three levels of caching. Explain why they are designed like this. [4 marks] In order to investigate...
-
Portray in words what transforms you would have to make to your execution to some degree (a) to accomplish this and remark on the benefits and detriments of this thought.You are approached to compose...
-
Let be an arbitrary operation in Problems 5259. Describe the operation for each problem. 5038; 70 2= 9; 901 = 10; 8 0 2 = 10; -
-
Discuss the two (2) sources of innovation classified as knowledge push and need pull. Provide an example of each classification and discuss two (2) driving factors that encouraged the development of...
-
Tami Tyler opened Tamis Creations, Inc., a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain on Ms....
-
Describe the Planning theme in a PRINCE2 project. AppendixLO1
-
Pams Creations had the following sales and purchase transactions during Year 2. Beginning inventory consisted of 60 items at $350 each. The company uses the FIFO cost flow assumption and keeps...
-
For the firm in Problem 6, suppose the book value of the debt issue is $135 million. In addition, the company has a second debt issue, a zero coupon bond with 12 years left to maturity; the book...
-
Gas A expands through an adiabatic turbine. The inlet stream fl ows in at 100 bar and 600 K while the outlet is at 20 bar and 445 K. Calculate the work produced by the turbine. The following data are...
-
We are interested in the thermodynamic properties of a strip of rubber as it is stretched (see below). Consider n moles of pure ethylene propylene rubber (EPR) that has an unstretched length z0. If...
-
A particular stream widens as it progresses downstream. Using your answers for parts (a) and (b), briefly describe the changes in discharge. (a) If the cross-sectional area of the stream is 1 m 2 and...
-
The Tokyo Olympics. After watching how the tokyo olympics became the most expensive summer game ever video answer the following questions. Q 3 : As you saw in the video, the capital investment a city...
-
write at least two paragraphs discussing the experiences of individuals who identify outside the traditional binary gender system (male/female.) Please explore the challenges they face and how...
-
Newly formed S&J Iron Corporation has 163,000 shares of $5 par common stock authorized. On March 1, Year 1, S&J Iron issued 9,000 shares of the stock for $12 per share. On May 2, the company issued...
-
Use the SMOKE for this question. The variable cigs is the number of cigarettes smoked per day. How many people in the sample do not smoke at all? What fraction of people claim to smoke 20 cigarettes...
-
Transcribed image text : Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit...
-
Economists refer to price segmentation as price discrimination. Does this mean that every price-segmentation technique is considered to be unfair?
-
In a paragraph of approximately 150-200 words, analyze a film or TV/Streaming Show poster of your choosing by focusing on the ways in which representations in the poster are gendered. Include an...
-
Self-assembled monolayers (SAMs) are receiving more attention than Longmuir-Blodgett (LB) films as starting points for nanofabrication. How do SAMs differ from LB films and why are SAMs more useful...
-
Explain the physical origins of surface activity by surfactant molecules.
-
Calculate the number-average molar mass and the mass-average molar mass of a mixture of two polymers, one having M = 62 kg mol-1 and the other M = 78 kg mol-1, with their amounts (numbers of moles)...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse
The Law StudentS Pocket Mentor From Surviving To Thriving 1st Edition - ISBN: 0735540349 - Free Book

Study smarter with the SolutionInn App


