The standard half-cell potential for the reduction of water to form hydroxyl ion in Table 9.1 is
Question:
The standard half-cell potential for the reduction of water to form hydroxyl ion in Table 9.1 is reported as -0.828 V. If instead we write the reaction: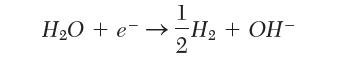
what should we use for the half-cell potential?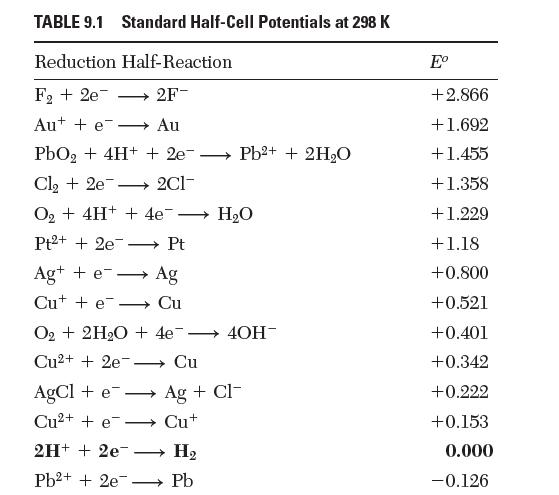
Transcribed Image Text:
1 HO +e=H + OH- 2H + OH-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Many important biological reactions involve electron transfer. Because the pH of bodily fluids is close to 7, the biological standard potential of an electrode, E*, is measured at pH = 7....
-
Portray in words what transforms you would have to make to your execution to some degree (a) to accomplish this and remark on the benefits and detriments of this thought.You are approached to compose...
-
"I'm not sure we should lay out $300,000 for that automated welding machine," said Jim Alder, president of the Superior Equipment Company. "That's a lot of money, and it would cost us $84,000 for...
-
A teacher has just given an algebra exam. What are some of the statistics she could compute?
-
______ refers to any work done for a company by people other than the company's employees and the country in which company located. A. off shoring B. Outsourcing C. In-house
-
A spaceship is initially at rest in the lab frame. At a given instant, it starts to accelerate. Let this happen when the lab clock reads t = 0 and the spaceship clock reads t0 = 0. The proper...
-
Life tests of cutting tools. Refer to the data on life tests of cutting tools, Exercise 11.52 (p. 636). a. Use a 90% confidence interval to estimate the mean useful life of a brand-A cutting tool...
-
Horace and Lee CPAs (H&L) is a medium-sized CPA firm that performs review and audit engagements for mostly privately held companies. H&L also has a tax group. H&L is considering accepting...
-
What Ex 6 . 1 4 Consumption ratios, achivity rates Bienestar Company produces two types of get - well cards: scented and regular. Drivers for the four activities are as follows: \ table [ [ , Scented...
-
Consider an electrochemical cell with a 1 m concentration of sodium ions and Al and Pt electrodes electrically connected together. Will the Al spontaneously dissolve into solution?
-
Consider the following liquid phase reactions: A plot of the concentrations in [mol/L], of A, B, and C in a constant-volume batch reactor operating isothermally at 500 K is shown in the following fi...
-
At the beginning of the summer, Brent Davis was looking for a way to earn money to pay for his college tuition in the fall. He decided to start a lawn service business in his neighborhood. To get the...
-
Write a java program that contain two overloaded methods that accepts two numbers or two characters representing a range example (11, 37) or (c, w) inputted by the user. The method generates a random...
-
Maggie could not conceive a child using natural means, so she sought out a woman who would donate an egg to be surgically implanted in Maggie. Which of the following items are deductible by Maggie in...
-
M corporation is subject to tax only in state b state b law provides for the use of federal taxable income before net operating loss and special deductions as the starting point for computing state...
-
Use Routh Criteria to determine the values of K needed for the system represented by the Characteristic Equation to be stable. (1 + K)s + (2K + 3)s + 2 3K = 0 Obtain the root locus plot for the...
-
Q7 a) Two forces equal to 2P and P act on a particle. If the first be doubled and second is increased by 12N, the direction of resultant remains unaltered. Find the value of P (5)
-
Determine taxable income in each of the following instances. Assume that the corporation is a C corporation and that book income is before any income tax expense. a. Book income of $50,000 including...
-
What recommendations would you make to Big Four firms to help them (1) avoid confrontations with governmental officials in an authoritarian society and (2) deal effectively with such confrontations...
-
Determine the DU for each of these structures: Jesen OCHCH3 c) Br COH -Og OCCH3 Acetylsalicylic acid (aspirin) b) N NH, O CH-CH-C-OH H Tryptophan (an amino acid)
-
What kinds of attractive intermolecular forces are found in each of these compounds? (a) CH 3 CH 2 CH 3 (b) CH 3 OCH 3 (c) CaCl 2 (d) CH 3 OH
-
Show the hydrogen bond that is present in liquid ammonia, NH 3 (l)
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...
-
help me A 35% discount on 3 smart phone amounts to $385. What is the phone's list price? Answer =$ (rounded to the nearest cent)

Study smarter with the SolutionInn App


