Ts diagrams for two reversible thermodynamic power cycles are shown in the following fi gure. Both cycles
Question:
Ts diagrams for two reversible thermodynamic power cycles are shown in the following fi gure.
Both cycles operate between a high temperature reservoir at 500 K and a low temperature reservoir at 300 K. The process on the left is the Carnot cycle described in Section 2.9. The process on the right is a Stirling cycle, which is similar to a Carnot cycle, except that the two steps (state 4 to state 1)
and (state 2 to state 3) are at constant volume. Which cycle, if either, has a greater effi ciency? Explain.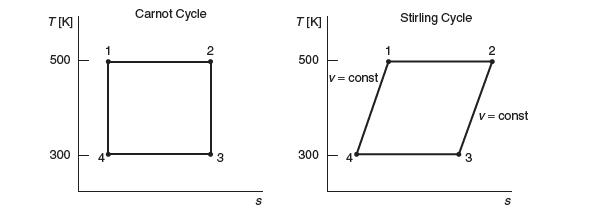
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





