Using data from Table 4.2, estimate the equilibrium bond length that would exist in a molecule of
Question:
Using data from Table 4.2, estimate the equilibrium bond length that would exist in a molecule of Xe2.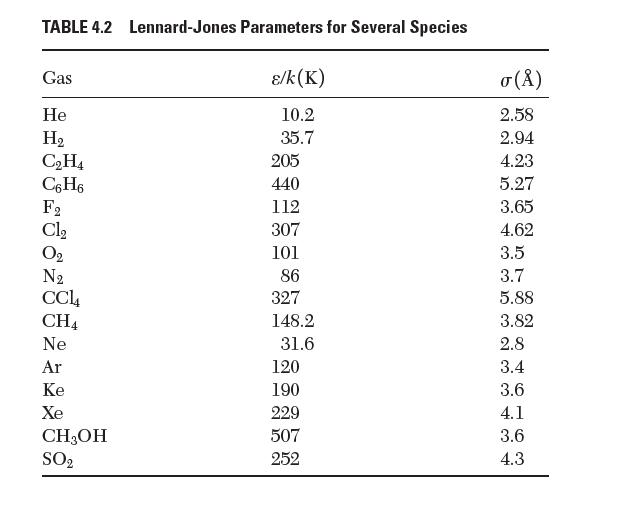
Transcribed Image Text:
TABLE 4.2 Lennard-Jones Parameters for Several Species /k(K) 10.2 35.7 Gas He H CH4 C6H6 288 28 Cl CH4 Ne Ar Ke Xe CH3OH SO 205 440 112 307 101 86 327 148.2 31.6 120 190 229 507 252 () 2.58 2.94 4.23 5.27 3.65 4.62 3.5 3.7 5.88 3.82 2.8 3.4 3.6 4.1 3.6 4.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Northern Virginia Community College HOW MUCH FINANCIAL RISK SHOULD YOU TAKE? Mark D. D'Antonio Nova Southeastern University FORT LAUDERDALE, FLORIDA, U.S.A. Abstract A successful retirement...
-
Using data from Table 8.4 on bond enthalpies, show that the more C---H bonds a molecule has compared to C-----O and O----H bonds, the more energy it can store.
-
PLEASE GIVE CORRECT ANSWERS Prove that the number of comparators in any sorting network is (n log n). [4 marks] (ii) What does Part (d)(i) imply in terms of the depth of any sorting network? [1 mark]...
-
1. By integrating Planck's codiation law over all wave hengths power radiated per square meter of a is given by: R(T) = (55) T" proof that the cavity's surface note: x= he AKT 2 xdx 3 % ex-1
-
John Timms is the sole owner of Sunshine Wholesale Traders, a company which buys fruit from farmers and sells it to supermarkets. All goods are collected from farms and delivered to supermarkets on...
-
The weights of a large number of miniature poodles are approximately normally distributed with a mean of 8 kilograms and a standard deviation of 0.9 kilogram. If measurements arc recorded to the...
-
C14.7. Thehigherthe anticipated return onnetoperating assets(RNOA) relative to theanticipated growth in net operating assets, the higher will be the unlevered priceto- book ratio. Is this correct?
-
The ownermanager of Good Guys Enterprises obtains utility from income (profit) and from having the firm behaves in a socially conscious manner, such as making charitable contributions or civic...
-
sent to the expertP 3 . 6 ( LO 3 , 4 ) , AP Hamilton Processing Company uses the weighted - average method and manufactures a single product an industrial carpet shampoo used by many universities....
-
Calculate the van der Waals parameter b for CH4, C6H6, and CH3OH. Based on these values, estimate the molecular diameter of each species. Compare the values obtained with those in Table 4.2. TABLE...
-
Calculate the bond strength in [eV] of a sodium ion in a crystal of NaCl. For the salt lattice: (a) Consider only the six nearest-neighbor Cl- ions. The Cl- ions are at a distance r = 2.76 . from the...
-
Gavin Petrosino, a building contractor, builds houses in tracts, often building as many as 20 homes simultaneously. Petrosino has budgeted costs for an expected number of houses in 20X0 as follows:...
-
From the perspective of organizational structure, design, and control, what have gone wrong at PNB? What factors have contributed to its current state of disarray? What should be done next to help...
-
Your goal is to advise the President on domestic economic policy. Role You are the chair of the Council of Economic Advisors (CEA) Audience Your audience is the President of the United States....
-
omework quiz 2.1 #1 stem plot The miles per gallon rating for 30 cars are shown below (lowest to highest). 19, 19, 19, 20, 21, 21, 25, 25, 25, 26, 26, 28, 29, 31, 31, 32, 32, 33, 34, 35, 36, 37, 37,...
-
Your answers are saved automatically. Question Completion Status: QUESTION 1 13 points Save Answer Library A computer memory manufacturer specifies that its memory chip stores data incorrectly an...
-
Analyze the possible reasons for and responses to Chung's request for a private office. What factors might impact Leary's decision? Identify at least two challenges and dilemmas in managing...
-
How do mechanistic models differ from interpretive models?
-
a. What is meant by the term tax haven? b. What are the desired characteristics for a country if it expects to be used as a tax haven? c. What are the advantages leading an MNE to use a tax haven...
-
Is either of the epoxides formed in the preceding reactions chiral? Is either epoxide optically active when prepared from the alkene by this method?
-
What is the principal organic product formed in the reaction of ethylene oxide with each of the following? (a) Sodium cyanide (NaCN) in aqueous ethanol (b) Sodium azide (NaN3) in aqueous ethanol (c)...
-
Given the starting material 1-methyl-1, 2-epoxycyclopentane, of absolute configuration as shown, decide which one of the compounds A through C correctly represents the product of its reaction with...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


