We can use the principles of phase equilibrium to learn about the stability of proteins in biological
Question:
We can use the principles of phase equilibrium to learn about the stability of proteins in biological systems. In this example, we consider the phase equilibrium of the protein lysozyme (l) between its native phase, n, and its denatured phase, d, where unfolding occurs.
The following thermochemical data are available. The heat capacity for each state is independent of temperature and is given by: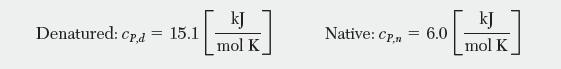
At 25°C, the enthalpy and entropy differences between native and denatured states are given by:
Answer the following questions:
A. At 25°C, which phase is stable? Justify your answer.
B. Plot the difference in Gibbs energy from the native state to the denatured state (gd - gn) versus temperature, T. On the plot label the following:
i. The heat-denaturation temperature, which is given as the temperature above which the native protein is no longer thermodynamically stable.
ii. The cold-denaturation temperature, which is given as the temperature below which the native protein is no longer thermodynamically stable.
iii. The temperature that the native state is most stable.
Step by Step Answer:






