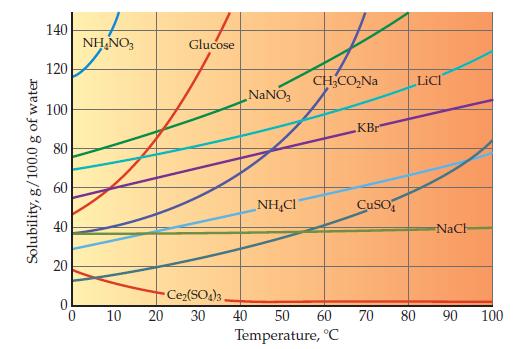
Examine the bottom graph on page 462 showing solubility in water as a function of temperature. What
Question:
Examine the bottom graph on page 462 showing solubility in water as a function of temperature. What is the trend for most of the ionic substances shown?
Data from Graph page 462
Transcribed Image Text:
Solubility, g/100.0 g of water 140 120 100 80 60 40 20 NH₂NO3 10 Glucose NaNO3 NHẠC CH₂CO₂Na Ce₂(SO4)3, 20 30 40 50 60 Temperature, °C KBr CuSO LICI NaCh 70 80 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The trend for most of the ionic substances shown in the bottom graph on page 462 is that their solub...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
One ionic compound in the bottom graph on page 462 shows almost no temperature dependence, and one clearly violates the general trend. Identify these two ionic compounds. Data from Graph page 462...
-
Use the bottom graph on page 462 to determine what mass of water is required to dissolve 50.0 g of NaNO 3 at 40.0 C. Graph from Page 462 Solubility, g/100.0 g of water 140 120 100 80 60 40 20 0 NHNO3...
-
A short-term study was conducted to investigate the effect of mean monthly daily temperature x1 and cost per kilowatt-hour x2 on the mean daily consumption of electricity (in kilowatt-hours, kWh) per...
-
A city levies property taxes of $2 billion in June 2015for its Fiscal year beginning July 1, 2015. The taxes are due by January 31, 2016. The following (in millions) indicates actual and anticipated...
-
JOURNAL ENTRIES FOR MATERIAL, LABOR, AND OVERHEAD Shiar Manufacturing had the following transactions during the month: (a) Purchased raw materials on account, $22,500. (b) Issued direct materials to...
-
Case Study Questions: 1. Based only on the information provided in the case study, use Michael Porters competitive forces model ( see figure 3.8 in Page 6 ) to analyze Bed Bath & Beyonds (BB&Bs)...
-
2. Voluntary health and welfare organizations: a Are required to use fund accounting principles to segregate unrestricted and restricted net assets b May report fund accounting information as...
-
Mr. R. Plane, a retired college administrator, consumes only grapes and the composite good Y (PY = $1). His income consists of $10,000/yr from social security, plus the proceeds from whatever he...
-
During Week 1, we will examine the various financial statements prepared by accountants. Review the balance sheet, income statement, and statement of cash flows and discuss the uses of the statements...
-
Aquatic life is often damaged when hot water is discharged from power stations into rivers and lakes. What might this have to do with gas solubility in water?
-
How is the medical condition known as the bends related to solubility?
-
A circular-motion addict of mass 80 kg rides a Ferris wheel around in a vertical circle of radius 10 m at a constant speed of 6.1m/s. (a) What is the period of the motion? What is the magnitude of...
-
Daisy Cakes website on YouTube: https://www.youtube.com/watch?v=AVM-RuLh2KI Daisy Cakes is looking for financing from the sharks to expand her business. You will realize that Kim, the founder of...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Laker Company reported the following January purchases and sales data for its only product. For specific identification, ending inventory consists of 280 units from the January 30 purchase, 5 units...
-
Compensation (wages) Income taxes withheld $ 36,600 7,680 FICA taxes at a 7.65% rate (no employee had reached the maximum). Required: A. Prepare the March 31, 2022 journal entry to record the payroll...
-
Process Costing and Spoilage Nation Lovers PLC produces several items to be used as replacement tools for various types of machineries. The product costing system for NL which is used as spare part...
-
Let A, B, and C denote n n matrices and assume that det A = -1, det B = 2, and det C= 3. Evaluate: det(B2C-1AB-1CT)
-
Repeat Exercise 16.6 using the t-test of the coefficient of correlation. Is this result identical to the one you produced in Exercise 16.6?
-
Use tabulated standard half-cell potentials to calculate the standard cell potential for the reaction in an electrochemical cell at 25 C: Zn 2+ (aq) + H 2 O 2 (aq) Zn (s) + O 2 (g) + 2 H + (aq)
-
Use the tabulated half-cell potentials to calculate K for the oxidation of nickel by chlorine: Cl 2 (g) + Ni (s) 2 Cl (aq) + Ni 2+ (aq)
-
An electrochemical cell is based on two half-reactions: Oxidation: Fe(s) Fe 2+ (aq, 0.010 M) + 2 e Reduction: Br 2 (l) + 2 e 2 Br (aq, 1.0 M) Compute the cell potential.
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


