The following is a two-step mechanism for how chlorine atoms in the upper atmosphere react with and
Question:
The following is a two-step mechanism for how chlorine atoms in the upper atmosphere react with and decompose ozone.
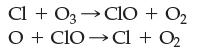
According to the mechanism, which is the intermediate and which is the catalyst? Justify your choices.
Transcribed Image Text:
Cl + O3 →CIO 03 + O₂ OCIO Cl + O₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
According to the image the chloride atom Cl is the catalyst in the twostep mechanism for how chlorin...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
A proposed two-step mechanism for the destruction of ozone in the upper atmosphere is a. What is the overall balanced equation for the ozone destruction reaction? b. Which species is a catalyst? c....
-
You are a contractor that has been asked to build an apartment complex. The associated AON Network diagram and the Time/Cost table are provided below. (Based on the table, you could crash activity B...
-
The following is a questionnaire to be completed by customers of the Hilltop Smoked Meat Co. Restaurant. Build a codebook that might be used to transfer raw answers from completed questionnaires to a...
-
Norma received a deficiency letter from the Internal Revenue Service. She appealed to the Independent Office of Appeals, and her appeal was denied. Now she wants to take the government to court....
-
What is a journal entry?
-
Consider the following correlation matrix: Describe how you would find out from the correlation matrix whether (a) There is perfect collinearity, (b) There is less than perfect collinearity, (c) The...
-
Rabato Corporation acquired merchandise on account from a foreign supplier on November 1, 2009, for 60,000 LCU (local currency units). It paid the foreign currency account payable on January 15,...
-
Terck, a leading pharmaceutical company, currently has a balance sheet that is as follows: The firms income statement looks as follows: Revenues.............. $1,000 Cost of goods sold (COGS)...
-
A health dinic invests $5,000,000 in a community health program. They expect an annual retum of 12.7% over the next 10 years, and the returns are compounded monthly. What will be the future value of...
-
A student claims that an endothermic reaction will always have a higher activation energy than an exothermic reaction, because an endothermic reaction ends up with the products at a higher energy...
-
Consider the decomposition of ozone (O 3 ) to oxygen (O 2 ). The rate law for this reaction is: Rate = k[O 3 ] 2 /[O 2 ]. How is the rate of this reaction affected by the concentration of oxygen?...
-
The mass center G of the 20-lb wheel is off center by 0.50 in. If G is in the position shown as the wheel rolls without slipping through the bottom of the circular path of 6-ft radius with an angular...
-
Units processed during September for material and conversion. Ask an instructor lock lock lock A 3 A copy Determine the cost per equivalent unit for material and conversion cost combined. copy...
-
12% of all college students volunteer their time. Is the percentage of college students who are volunteers different for students receiving financial aid? Of the 338 randomly selected students who...
-
Mervon Company has two operating departments: mixing and bottling. Mixing has 3 3 0 employees and Bottling has 2 2 0 employees. Indirect factory costs include administrative costs of $ 1 8 2 , 0 0 0...
-
XP Ltd. is a manufacturing company with high stock requirements. Management are currently considering their stockholding policy. The following information is available for one stock item, material...
-
Process Costing: weighted average method Required: make a cost of production report in good form. Cost of Production Report-Weighted Average First Dept- Gem Company applies 100% of materials at the...
-
The third matrix on the menu is just the identity matrix I. How do x and Ix compare geometrically as you rotate x around the unit circle? What can you conclude about the eigenvalues and eigenvectors...
-
Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [HO]. Be sure to enter Keq in proper scientific notation. (a) ammonia (acting as a base) reacts...
-
A rocket-powered sled of mass 3500 kg travels on a level snow-covered surface with an acceleration of +3.5 m/s 2 (Fig. P3.7). What are the magnitude and direction of the force on the sled? Figure...
-
Two balls are thrown from a tall bridge. One is thrown upward with an initial velocity +v 0 , while the other is thrown downward with an initial velocity -v 0 . Which one has the greater speed just...
-
Your car has a dead battery. It is initially at rest, and you push it along a level road with a force of 120 N, finding that it reaches a velocity of 2.0 m/s in 50 s. What is the mass of the car?...
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...
Beginners Blueprint For Real Estate Investing Success 1st Edition - ISBN: 979-8866680641 - Free Book

Study smarter with the SolutionInn App


