Which, if any, of these molecules are polar? For any molecule you classify as polar, show both
Question:
Which, if any, of these molecules are polar? For any molecule you classify as polar, show both the individual bond dipole moment vectors and the overall molecular dipole moment vector. Explain your answers.
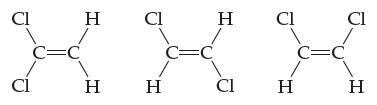
Transcribed Image Text:
Cl Cl C=C H H CI H C=C H CI Cl H C=C Cl H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Which if any of these molecules are polar The following molecules are polar Methanol CH3OH Water H2O Acetone C3H6O Ethanol C2H6O Individual bond dipol...View the full answer

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Draw two ammonia molecules in their three-dimensional shape and show how they would be attracted to each other. Show the partial charges and individual bond dipole moment vectors for both molecules,...
-
Consider the molecule N 2 O (connected NNO). (a) Draw the dot diagram. (b) Draw the molecules three-dimensional shape, and label the numeric value of all bond angles. (c) What is the shape of this...
-
Consider the molecule SiCl 4 . (a) Draw the dot diagram. (b) Draw the molecules three-dimensional shape, and label the numeric value of all bond angles. (c) What is the shape of this molecule? (d)...
-
On December 1, 2016, Masipag sold land in exchange for a P180,000 non-interest, 1-year promissory note. The 10% interest rate was going market rate for similar notes. Masipag had paid P66,000 to...
-
Why are there objections to using absorption costing when segment reports of profitability are being prepared?
-
Pamper Me Salon Inc.'s general ledger at April 30, 2017, included the following: Cash $5,000, Supplies $500, Equipment $24,000, Accounts Payable $2,100, Notes Payable $10,000, Unearned Service...
-
Fill in the following table for each of your companies. Stock P/E P/B P/S PEG Profit margin Return on divided by equity divided by P/S P/B
-
Pam runs a mail-order business for gym equipment. Annual demand for TricoFlexers is 16,000. The annual holding cost per unit is $2.50, and the cost to place an order is $50. What is the economic...
-
When you add multiple tables to a data model, what feature provides the ability to access data from all the tables in one Pivot Table? Building relationships. Calculated columns. Measures. Hierarchies
-
Draw a combined Lewis dot, molecular-shape diagram for each of the following species. Name each shape, and indicate whether the molecule or ion has a dipole moment. If so, draw the dipole moment...
-
Draw a combined Lewis dot, molecular-shape diagram for each of the following species. Name each shape, and indicate whether the molecule or ion has an overall dipole moment. If so, draw the dipole...
-
Refer to the revenue recognition practices of Qwest Communications outlined in Theory in Practice 1.1. Required a. Use the concept of relevance to argue that firms should record revenue as earned as...
-
Dr. Burgess oversees the pharmacy center within Hughes Regional Hospital. Dr. Burgess is planning on purchasing two medication dispensing units which she wants to pay back in a short-term period. The...
-
On January 1, 2021, Wetick Optometrists leased diagnostic equipment from Southern Corp., which had purchased the equipment at a cost of $1,831,401. The lease agreement specifies six annual payments...
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Employee Whatis late and Address Payroll information A -...
-
Image caption
-
Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that time has just flown by, over twenty-four years...
-
Change the following from Cartesian to cylindrical coordinates. a. (2, 2, 3) b. (43, -4, 6)
-
The cash records of Holly Company show the following four situations. 1. The June 30 bank reconciliation indicated that deposits in transit total $720. During July, the general ledger account Cash...
-
When 2-hepten-4-one is treated with LDA, a proton is removed from one of the gamma () positions. Identify which position is deprotonated, and explain why the proton is the most acidic proton in the...
-
When optically active (S)-2-methylcyclopentanone is treated with aqueous base, the compoundloses its optical activity. Explain this observation, and draw a mechanism that shows how racemization...
-
The racemization process described in the previous problem also occurs in acidic conditions. Draw a mechanism for the racemization process in aqueous acid.
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


