A sodium acetate solution contains 110 g of NaC 2 H 3 O 2 per 100 g
Question:
A sodium acetate solution contains 110 g of NaC2H3O2 per 100 g of water. Refer to Figure 13.6 and determine whether the solution is unsaturated, saturated, or supersaturated at each of the following temperatures.
(a) 50 °C
(b) 70 °C
(c) 90 °C.
Figure 13.6
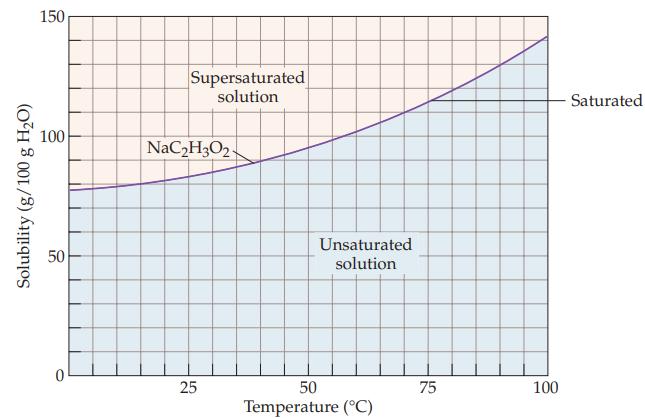
Transcribed Image Text:
Solubility (g/100 g H₂O) 150 100 50 0 Supersaturated solution NaC₂H302 25 Unsaturated solution 50 Temperature (°C) 75 100 Saturated
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a At 50 C the solubility of NaC 2 H 3 O 2 is about 97 g100 g water Since the solution ...View the full answer

Answered By

Lav Singh
I am a mathematics researcher working in the area of pure mathematics. I have published a few original articles in reputed journals. Besides research, I am passionate about teaching as well. I have been working on online-tutoring platforms for the past 4 years. I am also good at Latex and MS-word.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
On December 15, 20X2, you began as the new CFO of Trilogy Inc. Trilogy produces and manufactures stainless steel water bottles. You inherited the business (along with all of its problems) from your...
-
A sodium acetate solution contains 80 g of NaC 2 H 3 O 2 per 100 g water. Refer to Figure 13.6 and determine whether the solution is unsaturated, saturated, or supersaturated at each of the following...
-
A solution of 20.0 g KClO 4 in 500.0 g of water is brought to a temperature of 40 C. (a) Refer to Figure 14-10 and determine whether the solution is unsaturated or supersaturated at 40 C. (b)...
-
A room is 6 m by 5 m by 3 m. (a) If the air pressure in the room is 1 atm and the temperature is 300 K, find the number of moles of air in the room. (b) If the temperature rises by 5 K and the...
-
In what way do the adjustment and elimination entries for consolidation workpapers differ for the financial statement and trial balance approaches?
-
Using your example of audit sampling in the answer to R10.1, what items make up the population? What items are subject to being sampled? When the sample is complete, is the auditor drawing a...
-
The practice of charging all costs, both variable and fixed costs, to operation is (a) Absorption costing (b) Operation cost (c) Multiple cost (d) Process costing
-
Shortly after July 31, Towanda Corporation received a bank statement containing the following information: December cash transactions and balances on Towanda's records are shown in the following...
-
You can invest in taxable bonds that are paying a yield of 9.2 percent or a municipal bond paying a yield of 7.45 percent. Assume your marginal tax rate is 28 percent. a. Calculate the after-tax rate...
-
How is it possible to exceed the saturation of a solution and produce a supersaturated solution?
-
The solubility of nitrous oxide is 0.12 g/100 g water at 20 C and 1.00 atm. What is the partial pressure required to dissolve 0.55 g of the gas in 100 g of water at 20 C?
-
Heavenly Candy Company is considering purchasing a second chocolate dipping machine in order to expand its business. The information Heavenly has accumulated regarding the new machine is Cost of the...
-
Research a company that declared a 100% stock dividend or a two-for-one split Contrast the differences between a stock dividend and a stock split. Imagine that you are a stockholder in a company....
-
What are your ideas for Implementation and Assessing the Solution? How did you implement and assess the success? What should the time frame look like? What resources will be needed? What criteria...
-
What is an aesthetic question a viewer might ask about a work of art? 1 . What principles of design were used to make this work? 2 . What qualifies a functional object like this as a work of art? 3 ....
-
2. A phase diagram is shown below for an allotropic metal. Sketch and label possible Gibbs free energy curves for the 3 phases, as a function of temperature for the pressure indicated. Does the a or...
-
Recommend at least one (1) way a business with which you are familiar could use social media / buzz marketing to increase sales and awareness (e.g., social media awareness) of your...
-
For Magdalen Industries, compute the net income before taxes and net profit (or loss). Taxes for the year were $1 million. Calculate the interest coverage and net profit ratio. Is the interest...
-
Which of the followingcarbocations is the least stable? CH3CH2 . CH3CHCH3 CH3 I . CH3C0 T CH3 IV. V. CH3 CH3CCH2 CH3
-
Calculating Returns and Variability Using the following returns, calculate the arithmetic average returns, the variances, and the standard deviations for X and Y. Returns Year 6% 18% 24 39 13 -6 -20...
-
Calculating Returns and Variability Youve observed the following returns on Crash-n-Burn Computer s stock over the past five years: 2 percent, 8 percent, 24 percent, 19 percent, and 12 percent. a....
-
Calculating Real Returns and Risk Premiums for Problem 9, suppose the average inflation rate over this period was 3.5 percent and the average T-bill rate over the period was 4.2 percent. a. What was...
-
) A form used to organize and check data before preparing financial reports is known as a(n):A) statement of financial position.B) income statement.C) ledger. D) worksheet.2) Bringing account...
-
16) The entry to record the payment of office salaries would be: A) Debit Cash; Credit Salaries PayableB) Debit Cash; Credit Salaries ExpenseC) Debit Salaries Expense; Credit Accounts PayableD) Debit...
-
9) The general journal:A) is the book of original entry.B) is the book of final entry.C) contains account balances.D) is completed after the closing entries.10) The process of initially recording...

Study smarter with the SolutionInn App


