Balance each of the following chemical equations by inspection (a) Pb(s) + O(g) PbO(s) (b) LINO3(s)LiNO(s)
Question:
Balance each of the following chemical equations by inspection
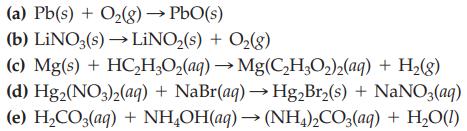
Transcribed Image Text:
(a) Pb(s) + O₂(g) → PbO(s) (b) LINO3(s)→→LiNO₂(s) + O₂(g) (c) Mg(s) + HC₂H₂O₂(aq) → Mg(C₂H₂O₂)2(aq) + H₂(g) (d) Hg2(NO3)2(aq) + NaBr(aq) → Hg₂Br₂(s) + NaNO3(aq) (e) H₂CO3(aq) + NH₂OH(aq) → (NH4)2CO3(aq) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Pbs O2g PbOs Count the number of atoms of each element on both sides of the equationThere is 1 lead Pb atom on the left and 1 lead Pb atom on the ri...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following chemical equations by inspection. (a) PCl 5 (s) + H 2 O(l) H 3 PO 4 (aq) + HCl(aq) (b) TiCl 4 (s) + H 2 O(g) TiO 2 (s) + HCl(g).
-
Balance each of the following chemical equations by inspection. (a) F 2 (g) + NaBr(aq) Br 2 (l) + NaF(aq) (b) Sb 2 S 3 (s) + HCl(aq) SbCl 3 (aq) + H 2 S(g).
-
Balance each of the following chemical equations by inspection. (a) FeO(l) + Al(l) Al 2 O 3 (l) + Fe(l) (b) MnO 2 (l) + Al(l) Al 2 O 3 (l) + Mn(l).
-
Compute the indicated quantities for the given homomorphism. Ker () for : S 3 Z 2 in Example 13.3 Data from Example 13.3 Let S n be the symmetric group on n letters, and let : S n Z 2 be defined by...
-
While examining cash receipts information, the accounting department determined the following information: opening cash balance $150, cash on hand $1,125.74, and cash sales per register tape $990.83....
-
What two factors directly affect the profitability of an FIs position in a foreign currency?
-
In which of the following areas does the IASB allow firms to choose between a benchmark treat ment and an allowed alternative treatment? LO4 a. Measuring property, plant, and equipment subsequent to...
-
On August 1, 2010, Cheryl Newsome established Titus Realty, which completed the following transactions during the month: a. Cheryl Newsome transferred cash from a personal bank account to an account...
-
Sales transactions: Journalize the following merchandise transactions a. Sold merchandise on account, $72,500 with terms, n/30. The cost of the goods sold was $43,500. b. Sold merchandise with a list...
-
Balance each of the following chemical equations by inspection. (a) Sn(s) + P(s) Sn3P(s) (b) Fe(CO3)3(s) FeO3(s) + CO(g) (c) Fe(s) + Cd(NO3)2(aq) Fe(NO3)3(aq) + Cd(s) (d) Co(NO3)2(aq) + HS(g) ...
-
Balance each of the following chemical equations by inspection. (a) Co(s) + O(g) CoO3(s) (b) LiClO3(s) LiCl(s) + O(g) (c) Cu(s) + AgCHO(aq) Cu(CHO)2(aq) + Ag(s) (d) Pb(NO3)2(aq) + LiCl(aq) PbCl(s)...
-
The chapter listed and defined four basic assumptions, four principles of measurement, and two exceptions. Review the annual report of MCI, and find at least one example of each of these ten...
-
The break even point of a company is $240 000. They sell their product at a markup of 30% and have variable expenses of 9% of sales. They currently make a profit of $10 500. They plan on reducing...
-
What kind of messages are young girls and boys receiving about whether their safety in relationships is valued are by their families and communities? Do we emphasize to our children (boys and girls)...
-
Below are the transactions for Oliver Printing, Incorporated for June, the first month of operations. June 1 Obtain a loan of $ 5 6 , 0 0 0 from the bank by signing a note. June 2 Issue common stock...
-
Assume an organization must invest $ 7 0 0 , 0 0 0 in fixed costs to produce a product that sells for $ 7 5 and requires $ 4 0 in variable costs to produce one unit. What is the organization s...
-
hotel delta marriott montreal What do you think is the value and purpose for the hotel brand choosing to make CSR an important part of their overall business strategy? What two recommendations based...
-
For a particle in a one-dimensional rectangular well, (a) Must there be at least one bound state? (b) Is ('' continuous at x = 0?
-
What are the six activities involved in the physical supply/distribution system?
-
Ratio Analysis the 2007 Annual Report of Eastman Kodak contains the following information. Compute the following ratios for Eastman Kodak for 2007. (a) Asset turnover ratio. (b) Rate of return on...
-
Book vs. Tax (MACRS Depreciation) Annunzio Enterprises purchased a delivery truck on January 1, 2010, at a cost of $41,000. The truck has a useful life of 7 years with an estimated salvage value of...
-
Book vs. Tax (MACRS Depreciation) Elwood Inc. purchased computer equipment on March 1, 2010, for $36,000. The computer equipment has a useful life of 10 years and a salvage value of $3,000. For tax...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


