Calculate the density of ammonia gas, NH 3 , at STP. (MM = 17.04 g/mol). Strategy Plan
Question:
Calculate the density of ammonia gas, NH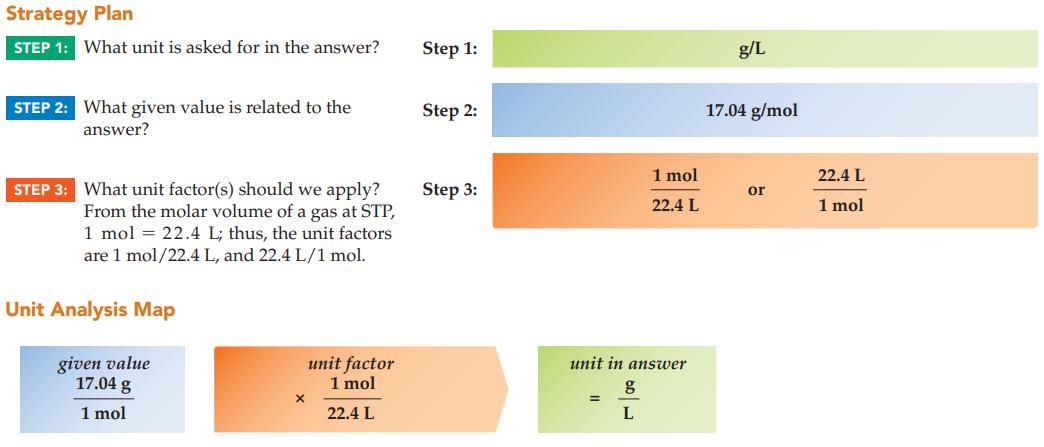 3, at STP. (MM = 17.04 g/mol).
3, at STP. (MM = 17.04 g/mol).
Transcribed Image Text:
Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is related to the answer? STEP 3: What unit factor(s) should we apply? From the molar volume of a gas at STP, 1 mol = 22.4 L; thus, the unit factors are 1 mol/22.4 L, and 22.4 L/1 mol. Unit Analysis Map given value 17.04 g 1 mol X unit factor 1 mol 22.4 L Step 1: Step 2: Step 3: unit in answer g = 1 mol 22.4 L L g/L 17.04 g/mol or 22.4 L 1 mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
We apply the unit factor ...View the full answer

Answered By

John Aketch
I am a dedicated person with high degree of professionalism, particularly in academic writing. My desire is to is to make students excel in their academic endeavor.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Calculate the density of oxygen at STP using the ideal gas law.
-
1. conditions. a) reaction: H Calculate the molar volume of H, gas under experimental b) -0.421 g Zn x _Lmol Zn Lmol H, 0.00644 mol 65.39 g Zn 1 mol Zn Second, the molar volume under experimental...
-
In Section 36.6 we saw that muons can reach the ground because of time dilation. But how do things appear in the muons reference frame, where the muons half-life is only 1.5 s? How can a muon travel...
-
Binger Inc. issues $3 million, 5-year, 10% bonds at 102, with interest payable on July 1 and January 1. The straight-line method is used to amortize bond premium. (a) Prepare the journal entry to...
-
Find the coefficient of determination r 2 and interpret the result. Use the data in the table, which shows the average annual salaries (both in thousands of dollars) for secondary and elementary...
-
Jamie Lee and Ross received a signed contract from the buyer accepting their $273,000 offer! The seller also agreed to pay two points toward Jamie Lee and Rosss mortgage. Calculate the benefit of...
-
Mervyns Fine Fashions has an average collection period of 50 days. The accounts receivable balance is $95,000. What is the value of its credit sales?
-
Consider the four capital budgeting projects listed below. The appropriate cost of capital is 8.5%. If these projects are mutually independent, and the company is not practicing capital rationing,...
-
Calculate the density of hydrogen sulfide gas, H 2 S, at STP.
-
State the number of moles represented by each of the following. (a) 6.02 x 10 23 atoms of sulfur, S (b) 6.02 x 10 23 molecules of sulfur dioxide, SO 2 .
-
Gam Co sells electronic equipment and is about to launch a new product onto the market. It needs to prepare its budget for the coming year and is trying to decide whether to launch the product at a...
-
Sketch the requested conic sections in Problems 14-23 using the definition. A parabola with the distance between the directrix and focus 1 unit
-
Why is the Rosenblum case a particularly important case in auditor liability?
-
Draw a population curve for a city whose growth rate is \(1.3 \%\) and whose present population is 53,000 . The equation is \[P=P_{0} e^{r t}\] Let \(t=0,10, \cdots, 50\) to help you find points for...
-
Draw a bar graph for each data set in Problems 32-35. Data set B Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 35 25 25 16 14 1 2 25 18 2 2...
-
For the four unrelated situations, A-D, below, calculate the unknown amounts indicated by the letters appearing in each column: B D Beginning Assets... Liabilities.. $40,000 $12,000 $28,000 $ (d)...
-
Let f = 2x2 - 5xyz + z2 - 1. Find grad f. Find 2f.
-
Suppose you are comparing just two means. Among the possible statistics you could use is the difference in means, the MAD, or the max min (the difference between the largest mean and the smallest...
-
What accounting treatment is normally given to the following items in accounting for plant assets? (a) Additions. (b) Major repairs. (c) Improvements and replacements.
-
New machinery, which replaced a number of employees, was installed and put in operation in the last month of the fiscal year. The employees had been dismissed after payment of an extra months wages,...
-
To what extent do you consider the following items to be proper costs of the fixed asset? Give reasons for your opinions. (a) Overhead of a business that builds its own equipment. (b) Cash discounts...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


