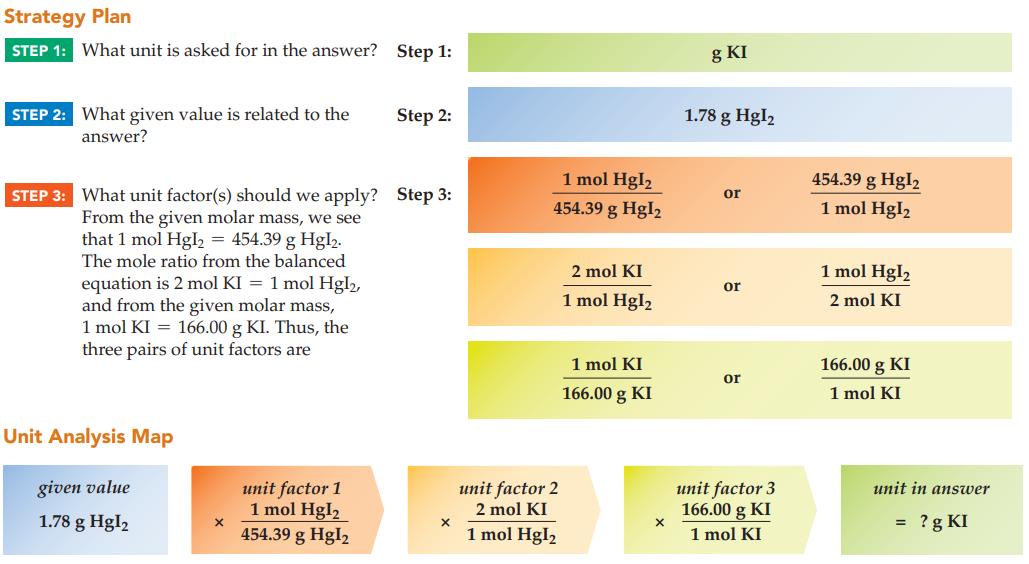
Calculate the mass of potassium iodide (166.00 g/mol) required to yield 1.78 g of mercury(II) iodide precipitate
Question:
Calculate the mass of potassium iodide (166.00 g/mol) required to yield 1.78 g of mercury(II) iodide precipitate (454.39 g/mol):
![]()
Transcribed Image Text:
2 KI(s) + Hg(NO3)2(ag) Hgl₂(s) + 2 KNO3(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
We select from each of the three pai...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Which is true regarding the bottom-up approach to budgeting expected project costs? A. The bottom-up approach allocates the overarching budget across the work packages. B. The bottom-up approach...
-
Calculate the mass of the precipitate formed when 2.27 L of 0.0820 M Ba(OH)2 are mixed with 3.06 L of 0.0664 M Na2SO4.
-
Mole Lab will rate answer Mole Laboratory Materials & Apparatus: Scale Graduated cylinder(s) Paper or plastic cups (8 oz or 16 oz) New container of small candy (jelly beans, Skittles, M&Ms,or...
-
Ebrahim Patel is a wholesaler who uses the periodic inventory system to account for inventory. Transactions for February: 1 Bought inventory from Rich Traders for R5 000 on credit. 2 Sold inventory...
-
Monsanto Company, a large chemical and fibers company, invested $37 million in state-of-the-art systems to improve process control, laboratory automation, and local area network (LAN) communications....
-
1. What is Friendly Candy Companys break-even point in boxes of candy for the current year? 2. What selling price per box must Friendly charge to cover the 15 percent increase in the cost of candy...
-
North Anglia Engineering Ltd (NAE), a manufacturing business, has recently obtained the financial statements of some of its industry rivals and has discovered that it holds, on average, twice the...
-
Peterson Company is preparing the annual financial statements dated December 31, 2010. Ending inventory information about the five major items stocked for regular sale follows: Required: Compute the...
-
Cost, Profit, and Investment Centers Analyze how cost center accounting practices in German companies differ from the practices in your current or previous organization or one with which you are...
-
In general, how many unit factors are required to solve a massmass stoichiometry problem?
-
What mass of oxygen gas is produced from decomposing 5.00 g of water (18.02 g/mol)? 2 H 2 O(l) 2 H 2 (g) + O 2 (g) (a) 2.22 g (b) 2.50 g (c) 4.44 g (d) 5.00 g (e) 8.88 g.
-
Several meteorites found in Antarctica are believed to have come from Mars, including the famous ALH84001 meteorite that was once thought to contain fossils of ancient life on Mars. Meteorites from...
-
1. The following data are available for JURIS DOCTOR CORP: Purchased raw materials from supplier amounting to P 40,000 on account.; During the month, raw materials costing P 30,000 were issued to...
-
The following financial information is available for Concord Corporation. (in millions) 2025 2024 Average common stockholders' equity $2,500 $2,600 Dividends declared for common stockholders 305 594...
-
Vecton's Bakery manufactures apple turnovers that passes through 4 sequential processes. Production data for February for Department 4 of the operation is as follows: Production data Units Opening...
-
write a code in java where we apply the sets and subsets to obtain functions as results. Let A= {1,2,3,4}, B={5,6,7,0}, C={8,9,10,11} and f: AB g:BC h: BC, all function are 1 to 1 a) Form the...
-
Consider the following LC-3 program. .ORIG x3000 LEA R1, LABEL LDR RO, R1, #231 LDI R1, LOCAL AND R3, R3, #0 LOOP AND R2, RO, R1 BRZ SHIFT ADD R3, R3, #1 SHIFT ADD R1, R1, R1 BRnp LOOP HALT LABEL...
-
Solve the nonlinear IVP dy/dt = 1(t+y), y(-1) = 0 by reinterpreting it with y as the independent variable and t as the dependent variable.
-
A. Select a recent issue (paper or online) of Report on Business Magazine, Canadian Business Magazine (online only), Bloomberg Businessweek, Fast Company, The Economist, or another business magazine....
-
Companies that make no variable-cost/fixed-cost distinctions must use absorption costing, and those that do make variable-cost/fixed-cost distinctions must use variable costing. Do you agree? Explain.
-
The main trouble with variable costing is that it ignores the increasing importance of fixed costs in manufacturing companies? Do you agree? Why?
-
Give an example of how, under absorption costing, operating income could fall even though the unit sales level rises.
-
explain the concept of Time Value of Money and provide and example. In addition to your discussion, please explain the differences between Stocks and Bonds
-
Wildhorse Inc. has just paid a dividend of $3.80. An analyst forecasts annual dividend growth of 9 percent for the next five years; then dividends will decrease by 1 percent per year in perpetuity....
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...

Study smarter with the SolutionInn App


