Carbon monoxide is produced in a blast furnace by passing oxygen gas over hot coal. How many
Question:
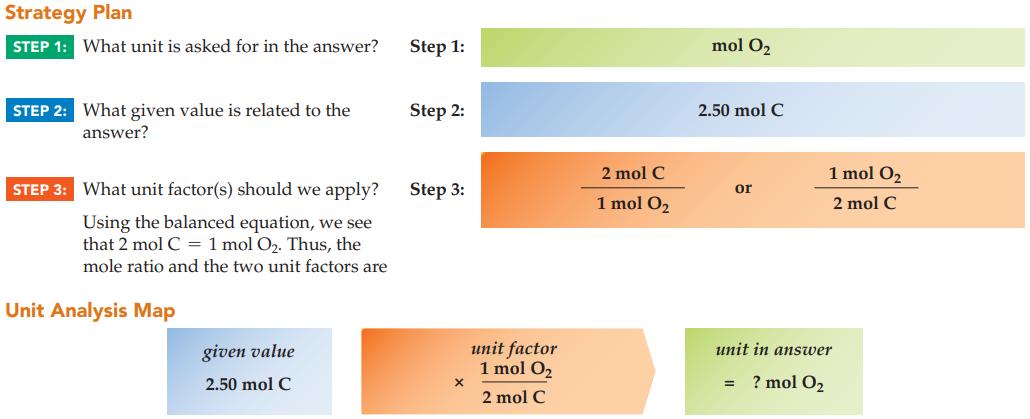
Carbon monoxide is produced in a blast furnace by passing oxygen gas over hot coal. How many moles of oxygen react with 2.50 mol of carbon, according to the balanced equation?
![]()
Transcribed Image Text:
2 C(s) + O₂(g) __A, 2 CO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
We select the unit fact...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Iron is produced from iron ore in a blast furnace by passing carbon monoxide gas through molten iron(III) oxide. The balanced equation is (a) How many moles of carbon monoxide react with 2.50 mol of...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
Common problems caused by a battery-operated and how you can solve it to help society and the environment?
-
Differentiate the function. f(x) = ln(x 2 + 10)
-
Childrens Hospital of the Kings Daughters Health System in Norfolk, Virginia, introduced a new budgeting method that allowed the hospitals annual plan to be updated for changes in operating plans....
-
Banyan Industries Limited (Banyan) manufactures various models of alternators, mainly for the North American automobile industry. The company, located in Canada, has grown steadily over the past 15...
-
Caring for a disabled or sick child can be taxing to say the least. Using the PewHealth dataset, create a crosstab that addresses this question: does caring for a sick child affect ones familys...
-
Bridget has a limited income and consumes only wine and cheese; her current consumption choice is four bottles of wine and 10 pounds of cheese. The price of wine is $10 per bottle, and the price of...
-
Please only answer this section... I just need row 2. Walsh Company manufactures and sells one product. The following information pertains to each of the company's first two years of operations:...
-
Oxygen gas in air reacts with 0.115 g hydrogen gas to give 1.024 g of water. Use the conservation of mass law to predict the mass of reacting oxygen. 2 H 2 (g) + O 2 (g) 2 H 2 O(l) (a) 0.115 g (b)...
-
Consider the general chemical equation: A + 2 B C + 2 D (a) How many moles of C are produced from 1 mol of A? (b) How many liters of gas B must react to give 2 L of gas D at the same temperature and...
-
Using equation (14.9), tabulate the entropy increase across a normal shock from Mach 1 to Mach 1.3 in Mach number increments of 0.02. What does the variation tell you about the nature of the...
-
The accounting records of the Eco Paper Company include the following information relating to the current year ended 31 March 2023: Materials 31 March 2023 $20,000 1 April 2022 $25,000 Work in...
-
The first read is an article on the development of money of a World War II prisoner-of-war, which was published in 1945. The second article was published in the opinion section of the New York Times...
-
Describe each Speaker's basic assumptions regarding employee motivation. That is, what are the underlying principles which guide how the Speaker treats his/her people (i.e., their direct report...
-
Find the area of the shaded region. The graph to the rate of IQ scores of adults, and those scores are normally distributed with the mean of 100 and a standard deviation of 15. x=81
-
In which scenario is Nikki showing resilience to stress? Nikki lost her job as an engineer 3 months ago. At first, she was depressed, but she realized she wanted to change career paths and decided to...
-
The rate of change dV/dt of the volume V of a melting snowball is proportional to its surface area, so for positive constant of proportionality k. (a) Explain how the relationship between the surface...
-
Clark, PA, has been engaged to perform the audit of Kent Ltd.s financial statements for the current year. Clark is about to commence auditing Kents employee pension expense. Her preliminary enquiries...
-
The trouble with discounted cash flow methods is that they ignore depreciation. Do you agree? Explain.
-
Lets be more practical. DCF is not the gospel. Managers should not become so enchanted with DCF that strategic considerations are overlooked. Do you agree? Explain.
-
All overhead costs are relevant in NPV analysis. Do you agree? Explain.
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


