Complete the following table and provide the missing information. Atomic Atomic Mass Number Number Notation Number Number
Question:
Complete the following table and provide the missing information.
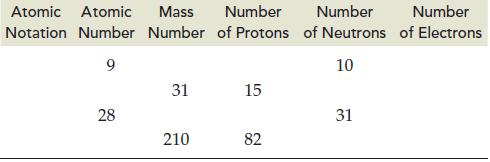
Transcribed Image Text:
Atomic Atomic Mass Number Number Notation Number Number of Protons of Neutrons 9 10 28 31 210 15 82 31 Number of Electrons
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To complete the following table we need to find the atom...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Complete the following table by computing the missing amounts (?) for the following independent cases. Principal Amount on Note Receivable a. $100,000 b. $50,000 Annual Interest Rate Time Period 6...
-
Complete the following table by calculating the missing entries. In each case indicate whether the solution is acidic or basic.
-
Complete the following table by calculating the missing entries and indicating whether the solution is acidic or basic.
-
In Exercises 1114, graph each equation in a rectangular coordinate system. If two functions are indicated, graph both in the same system. Then use your graphs to identify each relations domain and...
-
An annual report of the Maytag Corporation contained the following excerpt: The Company announced the restructuring of its major appliance operations in an effort to strengthen its position in the...
-
Write a summary of lessons given below https://accessdl.state.al.us/AventaCourses/access_courses/economics_ua_v20/02_unit/02-01/02-01_introduction.htm...
-
Sale prices of apartments. A Minneapolis, Minnesota, real-estate appraiser used regression analysis to explore the relationship between the sale prices of apartment buildings sold in Minneapolis and...
-
Which portfolio is better diversified, one that contains stock in a dental supply company and a candy company or one that contains stock in a dental supply company and a dairy product company?
-
You have the following information for Cullumber Company. Cullumber uses the periodic method of accounting for its inventory transactions. Cullumber only carries one brand and size of diamonds-all...
-
Draw a diagram of the arrangement of protons, neutrons, and electrons in an atom of each of the following isotopes. (a) 7 3 Li (b) 13 6 C (c) 16 8 O (d) 20 10 Ne
-
State the number of neutrons in an atom of each of the following isotopes. (a) Hydrogen-2 (b) Carbon-14 (c) Cobalt-60 (d) Iodine-131.
-
It is known from past experience that 7% of the tax bills are paid late. If 20,000 tax bills are sent out, approximate the probability that: (a) Less than 1350 are paid late. (b) 1480 or more are...
-
If Technical Specification 2 were reduced in the next design for this product, what would likely happen to customer opinion of Value Feature A? Quick Start QFD Matrix 2 Strong positive correlation...
-
Customer opinion of Value Feature B is most strongly correlated with what technical specification? Quick Start QFD Matrix 2 Strong positive correlation Some positive correlation == Strong negative...
-
Consider Quick Start QFD Matrix 1 above. Of the two value features, which do cus- tomers consider three times more important? Quick Start Quick Start QFD Matrix 1 = Strong positive correlation = Some...
-
Which technical spec can be most easily modified without changing current choices for the other two technical specs? Quick Start Quick Start QFD Matrix 1 = Strong positive correlation = Some positive...
-
Use Table A.1 to select 20 three-digit random numbers. Did any of the numbers occur more than once? How is it possible for a number to occur more than once? Make a stem-and-leaf plot of the numbers...
-
Give the products of the following reactions. If the products can exist as stereoisomers, show which stereoisomers are obtained. a. cis-2-pcntcne -4- HCI b. trans-2-pentene + HCI c. I...
-
Juanita owns a home in Richardson, TX. She purchases a Homeowners Policy (HO-3) from Farm State Ins. Co. The policy provides $100,000 in liability coverage (coverage E) and $5,000 in Med Pay coverage...
-
Since warrants lower the cost of the accompanying debt issue, shouldnt all debt be issued with warrants? What is the expected return to the holders of the bond with warrants (or the expected cost to...
-
What conversion price is built into the bond? MINI CASE Paul Duncan, financial manager of Edusoft Inc., is facing a dilemma. The firm was founded five years ago to provide educational software for...
-
What is the convertibles straight-debt value? What is the implied value of the convertibility feature? MINI CASE Paul Duncan, financial manager of Edusoft Inc., is facing a dilemma. The firm was...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


