Give the systematic IUPAC name for each of the following carboxylic acids. (a) (b) H-C-OH
Question:
Give the systematic IUPAC name for each of the following carboxylic acids.
(a)
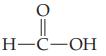
(b)
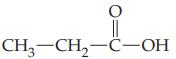
Transcribed Image Text:
H-C-OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a Meth...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Give an IUPAC systematic or common name for each of the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) CH3CN Cl NH2
-
The list of carboxylic acids in Table 19.1 is by no means exhaustive insofar as common names are concerned. Many others are known by their common names, a few of which follow. Give a systematic IUPAC...
-
Give an IUPAC systematic name for each of the following: (a) (b) (c) (d) (e) ONa Br OH C6Hs OH
-
Create the GUI in Fig. 14.2 (you do not have to provide functionality). Figure 14.2 Printer GUI Ok Printer. Bugalicious X9000 O Image O Selection Cancel Text O Current page Code ComboBox Setup....
-
Find the future values of the following ordinary annuities. a. FV of $400 each 6 months for 5 years at a nominal rate of 12%, compounded semiannually b. FV of $200 each 3 months for 5 years at a...
-
A 0.5-m 3 rigid tank contains nitrogen gas at 600 kPa and 300 K. Now the gas is compressed isothermally to a volume of 0.2 m 3 . The work done on the gas during this compression process is (a) 82 kJ...
-
Returns on common stocks. The total return on a stock is the change in its market price plus any dividend payments made. Total return is usually expressed as a percentage of the beginning price....
-
Exhibit 1: Prices and projected annual sales volumes for Sony PlayStation 3 and Microsoft Xbox 360 Elite Exhibit 2: Sony PlayStation 3 Production Costs per unit in dollars Exhibit 3: Microsoft Xbox...
-
On January 8, the end of the first weekly pay period of the year, Regis Company's employees earned $21,760 of office salaries and $70,840 of sales salaries. Withholdings from the employees' salaries...
-
An aromatic aldehyde and an aromatic ketone each have the molecular formula C 8 H 8 O. Draw the structure for each isomer.
-
An aldehyde and a ketone each have the molecular formula C 3 H 6 O. Draw the structure for each isomer.
-
Find K such that the function is a probability density function. f(x, y) = [ke-(x + y), x 0, y 0 0, elsewhere
-
In the global discourse on healthcare, the United States and England stand out as two contrasting models, each providing a distinct approach to addressing the challenges of cost , access, and...
-
2.A. Using the quotes below, answer the following questions. Exchange rate Bid Ask In New York, USD/EUR 1.2267 1.2875 In London, USD/GBP 1.6555 1.7334 2.A1. Calculate the EUR/GBP cross exchange...
-
Question 43 Part B Q1ii 20 points Save A a) A property is currently leased for $100,000 p.a. with fully recoverable outgoings. The lease has 3 years to run on the current (fixed) rent. The market...
-
Define HIPPA? What is the purpose of HIPPA? What are the 4 main rules of HIPPA?
-
Accounting for Inventories" Please respond to the following: As a Financial Accountant,determine the best type of income statement a retailer should use.Defend your suggestion. Analyze inventory...
-
You can use a single radio receiver to find the distance to a transmitter by measuring the strength of the signal. Suppose these approximate distances are measured with a receiver while you drive...
-
What mass of H2 will be produced when 122 g of Zn are reacted? Zn(s) + 2HCl(aq) ( ZnCl2(aq) + H2(g)
-
At the balance sheet date, a business owes a mortgage note payable of $360,000, the terms of which provide for monthly payments of $2,000. Explain how the liability should be classified on the...
-
Optimum Weight Co. offers personal weight reduction consulting services to individuals. After all the accounts have been closed on June 30, 2010, the end of the current fiscal year, the balances of...
-
List the errors you find in the following balance sheet. Prepare a corrected balancesheet. Cabana Services Co. Balance Sheet For the Year Ended August 31, 2010 Liabilities Assets Current liabilities:...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


