Give the systematic IUPAC name for each of the following aldehydes. (a) (b) O H-C-H
Question:
Give the systematic IUPAC name for each of the following aldehydes.
(a)
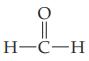
(b)
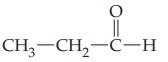
Transcribed Image Text:
O H-C-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Me...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Give an IUPAC systematic or common name for each of the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) CH3CN Cl NH2
-
Give an IUPAC systematic name for each of the following: (a) (b) (c) (d) (e) ONa Br OH C6Hs OH
-
Give the IUPAC name for each of the following hydrocarbons. a. b. c. d. , , CH3 CH3 CH3CCH2CH2CH2CCH3 CH3 CH3 CH CH2CHCH2CH2CH2CH3 CH2CH2CH3 CH3 CH CHCHCH2CH2CH2 CH3 CH2CH3
-
What other cost factors might you include in such an economic analysis? The proposed small office building in Example 3-2 has 24,000 net square feet of area heated by a natural gas furnace. The owner...
-
Find the future value of the following annuities. The first payment in these annuities is made at the end of Year 1, so they are ordinary annuities. a. $400 per year for 10 years at 10% b. $200 per...
-
On January 1, 20X0, Roland Inc. issued $125 million of 8% bonds at par. The bonds pay interest semiannually on June 30 and December 31 of each year, and they mature in 15 years. On December 31, 20X1...
-
Skewed left. Sketch a histogram for a distribution that is skewed to the left. Suppose that you and your friends emptied your pockets of coins and recorded the year marked on each coin. The...
-
In a laboratory experiment (designed to test a new brand), people are exposed to advertisements for a new brand and then are asked to buy a brand from that product class from a supermarket aisle that...
-
Mighty Company purchased a 60 percent interest in Lowly Company on January 1, 2020, for $461,400 in cash Lowly's book value at that date was reported as $645.000, and the fair value of the...
-
Ethylamine and diethylamine are soluble in water. Explain why triethylamine is only slightly soluble in water.
-
Ethylamine and propane have about the same molecular mass. Explain why the boiling point of ethylamine (17 C) is much higher than that of propane (-44 C).
-
Suppose Boeing faces the following demand curve for the monthly sales of its 787 aircraft: Q 120 0.5P Where Q is airplanes sold per month and P is the price in millions of dollars. The airplane...
-
4. What is the time complexity of the following procedure for in/2 to n do j 2 end for while (j
-
If the concentration of a constituent in the influent to the equalization basin is constant over the 24 h period, will the load of the constituent from the basin be constant? If the concentration of...
-
A three-phase transmission line of a 60 Hz circuit has a length of 370 km (230 miles). the conductors are of the 795,000cm (54/7) type with horizontal spacing of 25 feet between them. The load on the...
-
Simulate rolling a dice using Math.random() . Your roll function should allow the caller to specify any number of sides, but default to 6 if no side count is given: roll() assumes a 6 sided dice,...
-
Drama Read the excerpt from a play. Then, answer the question(s). (1) (2) Belle: Having trouble deciding what will make you look like both a power to be reckoned with and a fetching young lady while...
-
A panel of judges rate 20 science fair exhibits as shown. The judges decide that the top rating should be 100, so they add 6 points to each rating. a. What are the mean and the standard deviation of...
-
Which of the following is FALSE regarding the purchasing power parity (PPP). a. The PPP is a manifestation of the law of one price b. The PPP says that a country with a higher expected inflation can...
-
The Hometown Services Company is a financial planning services firm owned and operated by Jane Maines. As of March 31, 2010, the end of the current fiscal year, the accountant for The Hometown...
-
Lightworks Company maintains and repairs warning lights, such as those found on radio towers and lighthouses. Lightworks Company prepared the end-of-period spreadsheet (work sheet) shown below at...
-
Suspicions Company is an investigative services firm that is owned and operated by Curtis Graves. On November 30, 2010, the end of the current fiscal year, the accountant for Suspicions Company...
-
You are evaluating a new project for the firm you work for, a publicly listed firm. The firm typically finances new projects using the same mix of financing as in its capital structure, but this...
-
state, "The subscription price during a rights offering is normally r; lower ; lower r; higher er; higher than the rights-on price and
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...

Study smarter with the SolutionInn App


