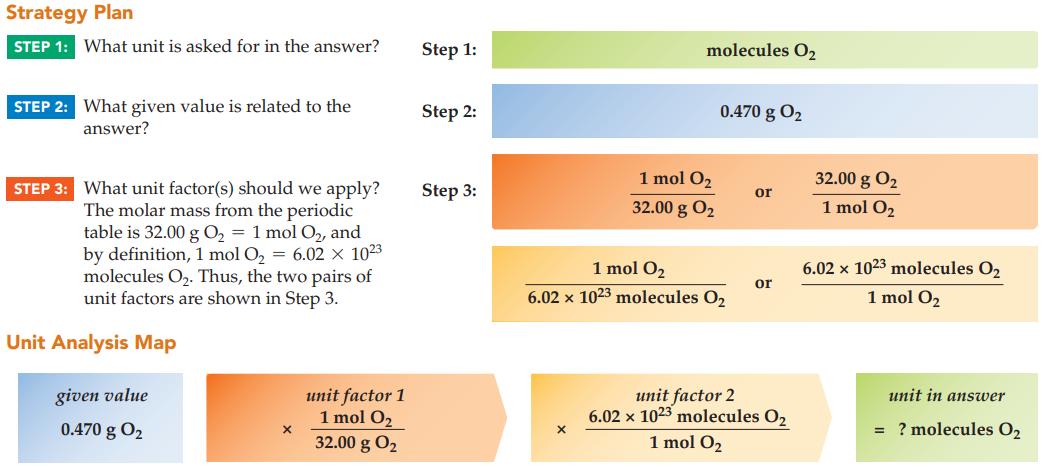
How many O 2 molecules are present in 0.470 g of oxygen gas? Strategy Plan STEP 1:
Question:
How many O2 molecules are present in 0.470 g of oxygen gas?
Transcribed Image Text:
Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is related to the answer? STEP 3: What unit factor(s) should we apply? The molar mass from the periodic table is 32.00 g O₂ = 1 mol O₂, and by definition, 1 mol O₂ = 6.02 × 1023 molecules O₂. Thus, the two pairs of unit factors are shown in Step 3. Unit Analysis Map given value 0.470 g 0₂ X unit factor 1 1 mol O₂ 32.00 g 0₂ Step 1: Step 2: Step 3: molecules O₂ X 1 mol O₂ 32.00 g 0₂ 0.470 g 0₂ 1 mol O₂ 6.02 x 1023 molecules O₂ or or unit factor 2 6.02 x 1023 molecules O2 1 mol O₂ 32.00 g 0₂ 1 mol O₂ 6.02 x 1023 molecules O₂ 1 mol O₂ unit in answer = ? molecules O₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
We apply the unit factor 1 mol O3200 g O ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Exactly 4.0 g of hydrogen gas combines with 32 g of oxygen gas according to the following reaction. 2H2 + O2 2H2O a. How many hydrogen molecules are required to completely react with 48 oxygen...
-
3.20 g of oxygen gas contains A. 0.200 mol O2 molecules B. 0.100 mol O atoms C. 0.100 mol O2 molecules D. 0.0500 mol O atoms
-
For divers going to great depths, the composition of the air in the tank must be modified. The ideal composition is to have approximately the same number of O2 molecules per unit volume as in surface...
-
Consider an ideal dual-loop heat-powered refrigeration cycle using R-134a as the working fluid, as shown in Fig. P9.135. Saturated vapor at 200 F leaves the boiler and expands in the turbine to the...
-
The following are selected transactions of Karolina Company. Karolina prepares financial statements quarterly. Jan. 2 Purchased merchandise on account from Pavel Company, $20,000, terms 2/10, n/30....
-
A Brazilian company called Netshoes completed its IPO on April 12, 2017, and listed on the NYSE. Netshoes sold 8,250,000 shares of stock to primary market investors at an IPO offer price of $18, with...
-
What is hedge accounting? LO9
-
University Ceramics manufactures plates, mugs, and steins that include the campus name and logo for sale in campus bookstores. The time required for each item to go through the two stages of...
-
Your real estate dream is to pay $400,000 for a house (all in cash) on Day 1. During the course of the following year, you will put $80,000 of improvements into the house. Each of the three years...
-
What is the mass of Avogadros number of ozone, O 3 , molecules?
-
How many molecules are found in 0.175 g of fluorine gas, F 2 ?
-
In an experiment to measure the Young modulus of glass, a student draws out a glass rod to form a fibre 0.800 m in length. Using a travelling microscope, she estimates its diameter to be 0.40 mm....
-
Convex Productions produces full-length motion pictures for distribution worldwide. Convex has just purchased the rights to a movie script entitled Native Sun, which it intends to develop as its next...
-
You are visiting the Engineering Office of Denton Hospital, as part of a consulting project. You notice some charts on one wall which look familiar to you: One of the employees notices you reading...
-
Richmond Clinic has obtained the following estimates for its costs of debt and equity at different capital structures: What is the firms optimal capital structure? (Hint: Calculate its corporate cost...
-
Suppose a sample yields estimates \(\widehat{\theta}_{1}=5, \widehat{\theta}_{2}=3\), se \(\left[\widehat{\theta}_{1} ight]=2\), and se \(\left[\widehat{\theta}_{2} ight]=1\) and the correlation...
-
Helium expands in a nozzle from \(0.8 \mathrm{MPa}, 500 \mathrm{~K}\), and negligible velocity to \(0.1 \mathrm{MPa}\). Calculate the throat and exit areas for a mass flow rate of \(0.34 \mathrm{~kg}...
-
In the Taylor series (4.85) of Prob. 4.1, let the point x = a be called xn (that is, xn ¡ a) and let s ¡ x - a = x - xn, so x = xn + s. (a) Use this notation to write (4.85) as f(xn + s)...
-
Consider model (9.18). What is the effect on the model parameter estimates, their standard errors, and the goodness-of-fit statistics when (a) The times at risk are doubled, but the numbers of deaths...
-
Purchases by Deferred Payment, Lump-Sum, and Nonmonetary Exchanges Klamath Company, a manufacturer of ballet shoes, is experiencing a period of sustained growth. In an effort to expand its production...
-
Acquisition, Improvements, and Sale of Realty Tonkawa Company purchased land for use as its corporate headquarters. A small factory that was on the land when it was purchased was torn down before...
-
Accounting for Self-Constructed Assets Troopers Medical Labs, Inc., began operations 5 years ago producing stetrics, a new type of instrument it hoped to sell to doctors, dentists, and hospitals. The...
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


