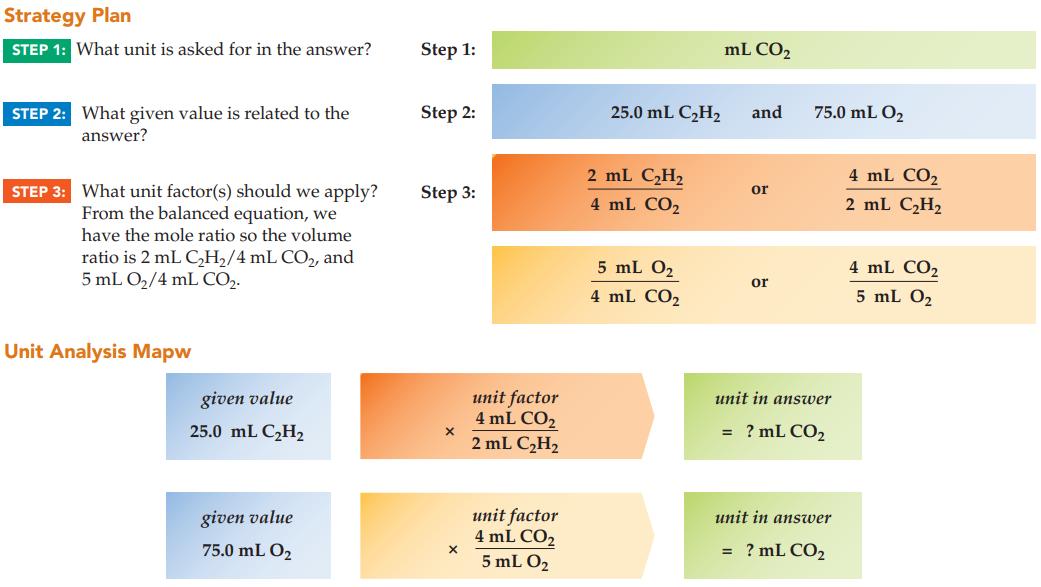
In oxyacetylene welding, acetylene reacts with oxygen to give carbon dioxide and water. If 25.0 mL of
Question:
In oxyacetylene welding, acetylene reacts with oxygen to give carbon dioxide and water. If 25.0 mL of C2H2 reacts with 75.0 mL of O2, what is the limiting reactant? Assuming constant conditions, what is the volume of CO2 produced?
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:





