Indicate the physical state (solid, liquid, gas) for each of the following at the given temperature. (a)
Question:
Indicate the physical state (solid, liquid, gas) for each of the following at the given temperature.
(a) Ne at -225 °C
(b) Ne at -255 °C
(c) Ar at -175 °C
(d) Ar at -200 °C
The melting points and boiling points for neon and argon are as follows.
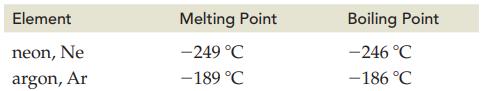
Transcribed Image Text:
Element neon, Ne argon, Ar Melting Point -249 °C -189 °C Boiling Point -246 °C -186 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
ANSWER To determine the physical state of a substance at a given temperature we ...View the full answer

Answered By

Jeff Omollo
As an educator I have had the opportunity to work with students of all ages and backgrounds. Throughout my career, I have developed a teaching style that encourages student engagement and promotes active learning. My education and tutoring skills has enabled me to empower students to become lifelong learners.
5.00+
5+ Reviews
52+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Classify each of the following materials as falling into one of the categories listed in Table 12.2. What particles make up these solids, and what are the forces of attraction between particles? Give...
-
Classify each of the following materials as falling into one of the categories listed in Table 12.2. What particles make up these solids and what are the forces of attraction between particles? Give...
-
Indicate the physical state (solid, liquid, or gas) for each of the following at the given temperature. (a) H 2 O at -10 C (b) H 2 O at 110 C (c) NH 3 at -100C (d) NH 3 at -50 C The melting points...
-
Scott and Associates, Inc., is an accounting firm that has three new clients. Project leaders will be assigned to the three clients. Based on the different backgrounds and experiences of the leaders,...
-
Assuming the same information as for Problem 21-2, suppose Hastings will increase Vandells level of debt at the end of Year 3 to $30.6 million so that the target capital structure is now 45% debt....
-
If a person promises to give you a gift, there is usually no consideration. The person can change his mind and decide not to give you the present, and there is nothing you can do about it. But if a...
-
What is meant by the term tracking the forecast? In which two ways can forecasts go wrong? LO.1
-
Hector Francisco is a successful businessman in Atlanta. The box-manufacturing firm he and his wife, Judy, founded several years ago has prospered. Because he is self employed, Hector is building his...
-
The accountant of Whack Limited has presented you with the following information relating to its financial year ended 3 1 December 2 0 2 0 : 1 . Profit for the year is R 4 0 0 0 0 0 . This profit has...
-
Classify each of the following crystalline solids as ionic, molecular, or metallic: (a) Iodine, I 2 (b) Silver iodide, AgI.
-
Classify each of the following crystalline solids as ionic, molecular, or metallic: (a) Nickel, Ni (b) Nickel(II) oxide, NiO.
-
As an investor purchases more shares over time, the time-weighted rate of return __________.
-
9. [10] Suppose that B and W are BMs and that they are correlated with correlation coefficient P (-1, 1) in the sense that the correlation coefficient between Bt and Wt for all t>0. Then we can...
-
You have just incorporated and started your business. Your corporate pre-tax profit is $40,000. This is your only source of income. This income is eligible for the Small Business Deduction and is...
-
4. Provide the information requested in the statements below: a) Find and draw all C's that do not contain H's (if any). For this, redraw the structure where you show the d ('s). N b) Find and draw...
-
Suppose that f(x) = 8x + 5. (A) Find the slope of the line tangent to f(x) at x = 7. (B) Find the instantaneous rate of change of f(x) at x = -7. C) Find the equation of the line tangent to f(x) at x...
-
Whichof the following regarding the relationship between business risk and financial risk is least accurate based on our discussions in class? A. Business risk represents uncertainty caused by...
-
A firm is considering three mutually exclusive alternatives as part of a production improvement program. The alternatives are as follows: For each alternative, the salvage value at the end of-...
-
In the busy port of Chennai, India, the number of containers loaded onto ships during a 15-week period is as follows: 1. Develop a linear trend equation to forecast container loadings. 2. Using the...
-
Figure shows that r D increases as the debt?equity ratio increases. In MM?s world r E also increases but at a declining rate. Explain why. Redraw Figure, showing how r D and change for increasingly...
-
Imagine a firm that is expected to produce a level stream of operating profits. As leverage is increased, what happens to (a) The ratio of the market value of the equity to income after interest? (b)...
-
Archimedes Levers is financed by a mixture of debt and equity. You have the following information about its cost of capital: Can you fill in the blanks? 12% 'D = 12% TE = BE = 1.5 10% BA D/V = .5 Bo...
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer

Study smarter with the SolutionInn App


