Nitric acid is prepared industrially by the conversion of ammonia. There are three steps in the conversion,
Question:
Nitric acid is prepared industrially by the conversion of ammonia. There are three steps in the conversion, which is referred to as the Ostwald Process.
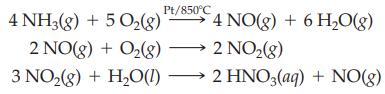
What is the mass of HNO3 (63.02 g/mol) produced, assuming 10.0 L of NH3 at STP is completely converted to nitric acid?
Transcribed Image Text:
Pt/850°C 4 NO(g) + 6 H₂O(g) 4 NH3(g) + 5 O₂(g) 2 NO(g) + O₂(g) →→→ 2 NO₂(g) 3 NO₂(g) + H₂O(l) →→→ 2 HNO3(aq) + NO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
We can plan our strategy for the relationship of NH 3 to HNO 3 as follows L NH 3 mol NH 3 mol ...View the full answer

Answered By

Subash Murugaih
I am leading expert in this web site couple of years and My clients are much happy with my works and services.
4.60+
309+ Reviews
539+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Ammonia is prepared industrially by the reaction: N2(g) + 3H2(g) 2NH3(g) for the reaction: H = 92.2 kJ and K (at 25C) = 4.0 108. When the temperature of the reaction is increased to 500C, which of...
-
Various uses for nitric acid are given in Problem 6.43, along with information about how this important chemical is synthesized industrially. The key reactions are oxidations of ammonia to nitric...
-
Nitric acid is produced commercially by the Ostwald process, represented by the following equations: 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) 2NO(g) + O2(g) 2NO2(g) 3NO2(g) + H2O(l) 2HNO3(aq) + NO(g)...
-
Consider : You have been asked to evaluate whether your organization's current pay structure makes sense in view of what competing - address the following: How would you determine what organizations...
-
Radon Homes current EPS is $6.50. It was $4.42 five years ago. The company pays out 40% of its earnings as dividends, and the stock sells for $36. a. Calculate the historical growth rate in earnings....
-
In this exercise, you modify the highest number program from the chapter. If necessary, create a new project named ModifyThis20 Project and save it in the Cpp8\Chap11 folder. Enter the C++...
-
What types of governmental actions should we be concerned about as we evaluate a country?
-
Using the data in BE4-6, journalize and post the entry on July 1 and the adjusting entry on December 31 for Craig Insurance Co. Craig uses the accounts Unearned Service Revenue and Service Revenue.
-
Partially completed T - accounts and additional information for Cardinals, Inc., for the month of November appear as follows. \ table [ [ Materials Inventory,Work - in - Process Inventory ] , [ B B (...
-
State the basic metric system unit for each of the following physical quantities. (a) Length (b) Mass (c) Volume (d) Time (e) Temperature (f) Heat energy.
-
Given 50.0 mL of 0.500 M hydrochloric acid, calculate each of the following: (a) Grams of HCl gas dissolved in the acid solution (b) Liters of HCl gas (at STP) dissolved in the solution (c) Molecules...
-
Before Miller Cereals can introduce the new cereal, the board of directors has to give their approval. The marketing manager really wants to introduce the new product and believes (honestly) that it...
-
Critical Values. In Exercises 41-44, find the indicated critical value. Round results to two decimal places. 41. Z0.25 42. Z090 43. Z0.02 44. Z0.05
-
Case Study X Ltd. has 10 lakhs equity shares outstanding at the beginning of the accounting year 2016. The appropriate P/E ratio for the industry in which D Ltd. is 8.35. The earnings per share is...
-
Notation of 0 + Using the same survey described in Exercise 1, the probability of randomly selecting 50 speaking characters from movies and getting 40 females is expressed as 0+. Does 0+ indicate...
-
A simple random sample of 10 pages from a dictionary is obtained. The numbers of words defined on those pages are found, with the results n = 10, x = 66.4 words, s = 16.6 words. Given that this...
-
Question 3 58.5 Average global temperature 1880-2013 58.0 $ 57.5 57.0 56.5 1880 1900 1920 1940 1960 1980 2000 2020 Year The graph above indicates that global temperatures have Ovaried randomly over...
-
An object is dropped from the top of a building into a pool of water at ground level. There is a splash 6.8 s after the object is dropped. How high is the building in meters? In feet?
-
Consider the setup in Problem 16. Show that the relative speed of the ball and the point of contact on the stick is the same before and immediately after the collision. (This result is analogous to...
-
Journal entries (continuation of 11-16). Refer to requirement 2 of Exercise 17-16. Prepare summary journal entries for the use of direct materials and incurrence of conversion costs. Also prepare a...
-
Zero beginning inventory, materials introduced in middle of process. Roary Chemicals has a Mixing Department and a Refining Department. Its process-costing system in the Mixing Department has two...
-
Weighted-average method, equivalent units. Consider the following data for the Assembly Division of Fenton Watches, Inc.: The Assembly Division uses the weighted-average method of process costing.
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


