Predict the ionic charge for a phosphorus ion, P ? , based on the position of the
Question:
Predict the ionic charge for a phosphorus ion, P?–, based on the position of the element in the periodic table.
Periodic Table:
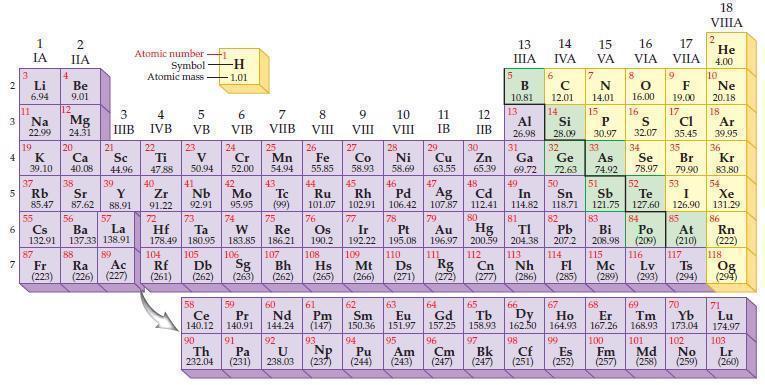
Transcribed Image Text:
2 3 4 15 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 Rb R 4 87 2 IIA Be 9.01 12 Mg 24.31 K Ca Sc 39.10 40.08 44.96 20 38 21 3 IIIB 39 Sr Y 85.47 87.62 88.91 57 55 56 La Cs Ba 132.91 137.33 138.91 88 Fr Ac Ra (223) (226) (227) 89 Atomic number Symbol Atomic mass 4 IVB 22 Ti 47.88 40 Zr 2 91.22 72 5 VB 104 23 V 50.94 41 73 Hf Ta 178.49 180.95 105 Rf Db (261) (262) -H -1.01 6 VIB Nb Mo 92.91 95.95 90 24 Cr 52.00 42 74 106 Sg (263) 58 Pr Ce 140.12 140.91 91 59 7 VIIB Th Pa 232.04 (231) 25 W Re 183.85 186.21 Mn 54.94 43. Tc (99) 75 107 Bh (262) 60 Nd 144.24 92 U 238.03 8 VIII 26 Fe 55.85 76 Os 190.2 61 Pm (147) 93 Np 9 VIII (237) 10 VIII 11 IB 12 IIB 27 28 29 Zn Co Ni Cu 58.93 58.69 63.55 65.39 47 44 49 45 46 48 Ru Rh Pd Cd In 101.07 102.91 106.42 107.87 112.41 114.82 Ag 77 78 79 80 Ir Pt 192.22 195.08 109 110 Hs Mt Ds Rg Cn (265) (266) (271) (272) (277) Au Hg 196.97 200.59 111 112 108 30 5 13 ΠΙΑ B 10.81 13 6 14 IVA с 12.01 14 81 82 TI Pb 204.38 207.2 113 114 Nh Fl (286) (285) 7 66 Ho 62 63 64 65 Sm Eu Gd Tb Dy 150.36 151.97 157.25 158.93 162.50 164.93 99 94 95 96 97 Pu Am Cm Bk (244) (243) (247) (247) 15 VA 67 N 14.01 Ne 20.18 18 Al Si S Ar 32.07 34 39,95 36 31 Kr 83.80 54 53 26.98 28.09 32 33 Ga Ge As Se 69.72 72.63 74.92 78.97 50 51 52 Sn Sb Te I Xe 118.71 121.75 127.60 126.90 131.29 83 84 85 86 Bi Po At Rn 208.98 (209) (210) (222) 115 116 117 118 Lv Ts (289) (293) (294) Mc Og (294) 15 16 17 VIA VILA P 30.97 8 O 16.00 9 F 19.00 17 Cl 35.45 16 35 68 69 70 Er Tm Yb 167.26 168.93 173.04 100 101 102 Cf Es Fm Md No (251) (252) (257) 98 (258) (259) Br 79.90 18 VIIIA 2 He 4.00 10 71 Lu 174.97 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Omar ELmoursi
I'm Omar, I have Bachelor degree in Business and Finance, My unique approach is to help students with questions and assignments, I can teach Business, Math, Accounting, Managerial Accounting, Economy, Human resources management, organizational behavior, project management, I have experience dealing with different types of students and teach them how to deal with different types of exercises.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Predict the ionic charge for a chlorine ion, Cl ?- , based on the position of the element in the periodic table. Periodic Table: 2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4 2 IIA 87 Be 9.01 12 K...
-
Predict the ionic charge for a sodium ion, Na ?+ , based on the position of the element in the periodic table. Periodic Table: 2 3 4 5 6 7 Li 6.94 11 Na 22.99 19 1 IA K 39.10 37 55 4 2 IIA 87 Be 9.01...
-
Predict the ionic charge for an aluminum ion, Al ?+ , based on the position of the element in the periodic table. Periodic Table: 2 3 4 15 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 Rb R 4 87 2 IIA Be...
-
The number of desks in one row is 5d + 2. How many desks are there in a room of 2d - 1 rows if they are arranged in a rectangular arrangement?
-
Describe the dual-track system used in federal agency accounting. Compare this to the system by the same name used in the discussion of state and local government reporting under GASB standards in...
-
Calculate the magnitude and direction of the magnetic field at point P due to the current in the semicircular section of wire shown in Fig. E28.34. Figure e28.34 R P
-
Learn how to assess the true potential of emerging markets. L01
-
An overhead door is guided by wheels at A and B that roll in horizontal and vertical tracks. Knowing that when en = 40o the velocity of wheel B is 0.6 m/s upward, determine (a) The angular velocity...
-
there are 4 parts to this question, what is the best way to get the answers to all parts without having to send 4 different pictures? Frugal Trailers' job cost records yielded the following...
-
What is the term for the elements that belong to Groups IIIBIIB (Groups 312)?
-
Predict the common ionic charge for Group IA/1 elements; Group IIA/2 elements; Group III/13 elements.
-
Suppose a maternal effect gene exists as a normal dominant allele and an abnormal recessive allele. A mother who is phenotypically abnormal produces all normal offspring. Explain the genotype of the...
-
Use least square regression to fit a straight line to the following data taken from the conductance (S/m) of a material with respect to temperature (C) of a composite material used to absorb heat....
-
A pile group consists of nine friction piles in clay soil (see Figure 10-40). The diameter of each pile is 16 in., and the embedded length is 30 ft each. Center-to-center pile spacing is 4 ft. Soil...
-
The rigid bar EBC is supported by two links AB and CD as shown in Figure 1. The Link AB is made of aluminum (E = 70 GPa) and the link CD is made of steel (E = 200 GPa). Both links have a Width = 30...
-
a well-insulated storage tank was pressurized under ideal gas conditions by air flowing into the tank. We used the first law to estimate the final temperature of the gas in the tank, Tf,tank- = We...
-
Transportation of natural gas is commonly done via pipelinesacross long distances. A company uses a 0.6-m diameter pipe totransport natural gas. Then pumping stations are located atdifferent points...
-
How do direct materials variances and direct labor cost variances differ from overhead cost variances?
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
Assume that IBM leased equipment that was carried at a cost of $150,000 to Sharon Swander Company. The term of the lease is 6 years beginning January 1, 2011, with equal rental payments of $30,044 at...
-
Use the information for IBM from BE21-6. Assume the direct-financing lease was recorded at a present value of $150,000. Prepare IBMs December 31, 2011, entry to record interest.
-
Jennifer Brent Corporation owns equipment that cost $80,000 and has a useful life of 8 years with no salvage value . On January 1, 2011, Jennifer Brent leases the equipment to Donna Havaci Inc. for...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


