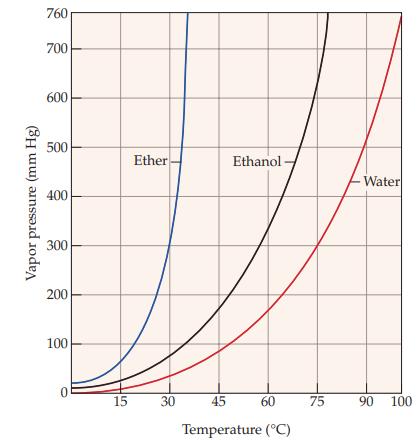
Refer to Figure 11.5 and determine the approximate boiling point of ether. Figure 11.5 Vapor pressure (mm
Question:
Refer to Figure 11.5 and determine the approximate boiling point of ether.
Figure 11.5
Transcribed Image Text:
Vapor pressure (mm Hg) 760 700 600 500 400 300 200 100 0 15 Ether 30 Ethanol 45 60 Temperature (°C) 75 > -Water 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The normal boiling point of a liquid is the temperature at which th...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 11.5 and determine the approximate boiling point of ethanol. Figure 11.5 Vapor pressure (mm Hg) 760 700 600 500 400 300 200 100 15 Ether 30 Ethanol 45 60 Temperature (C) 75 Water 90...
-
The warranty and customer service that come with the purchase of a car, are part of its _______product. Multiple Choice durable branded core actual augmented
-
Predict the approximate boiling point of (a) Ethylbenzene (b) Propylbenzene (c) P-xylene
-
A group of fraud examiners is coordinating an investigation at a local law firm. Several lawyers at the firm are suspected of overbilling clients, possibly creating fake client accounts, and then...
-
Nichols Corporations value of operations is equal to $500 million after a recapitalization (the firm had no debt before the recap). It raised $200 million in new debt and used this to buy back stock....
-
President Obama and others have called for repeal of the LIFO method for tax purposes. Conduct an internet search to find arguments for and against this proposal. Prepare a summary of these arguments.
-
Using exponential smoothing, calculate the forecasts for months 2, 3, 4, 5, and 6. The smoothing constant is 0.2, and the old forecast for month 1 is 245. Month Actual Demand Forecast Demand 1 260 2...
-
Reco Corp. is expected to pay a dividend of $2.25 next year. The forecast for the stock price a year from now is $37.50. If the required rate of return is 14 percent, what is the current stock price?...
-
Question 7 Jackson Company manufacturas chave the company reizes anunverble material price for the mother which of the towing condition is NECESAREY TRULY O A The company purchased more material than...
-
Which of the following is an observed property of liquids? (a) Liquids are more dense than gases. (b) Liquids expand and compress significantly. (c) Liquids have a fixed shape and variable volume....
-
Which of the following liquids has the stronger intermolecular attraction between molecules: CH 3 CH 2 OH or CH 3 OCH 3 ?
-
Consider a mixture of 50 mol% n-pentane and 50 mol% n-butane at 15 bar. (a) What is the dew temperature? What is the composition of the first drop of liquid? (b) At what temperature is the vapor...
-
Drs. Draper and Keys run a partnership family medical practice in Brownsville, Texas. While the practice is profitable, both physicians are making payments on heavy debt loads for student loans that...
-
Sweetlip Ltd and Warehou Ltd are two family-owned flax-producing companies in New Zealand. Sweetlip Ltd is owned by the Wood family and the Bradbury family owns Warehou Ltd. The Wood family has only...
-
Small Sample Weights of M&M plain candies are normally distributed. Twelve M&M plain candies are randomly selected and weighed, and then the mean of this sample is calculated. Is it correct to...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
The Tokyo Olympics After watching How the Tokyo Olympics Became the Most Expensive Summer Game Ever video answer the following questions. * * The numbers can be made up . I just need help with an...
-
Three mutually exclusive alternatives are being considered. Each alternative has a 20-year useful life with no salvage value . If the minimum attractive rate of return is 7%, which alternative should...
-
Suppose the index goes to 18 percent in year 5. What is the effective cost of the unrestricted ARM?
-
Curtis Bog, chief financial officer of Sphagnum Paper Corporation, is reviewing a consultant?s analysis of Sphagnum?s weighted-average cost of capital. The consultant proposes Mr. Bog wants to check...
-
Nevada Hydro is 40 percent debt-financed and has a weighted-average cost of capital of 9.7 percent: Banker?s Tryst Company is advising Nevada Hydro to issue $75 million of preferred stock at a...
-
Sometimes APV is particularly useful in international capital investment decisions. What kinds of tax or financing side effects are encountered in international projects?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


