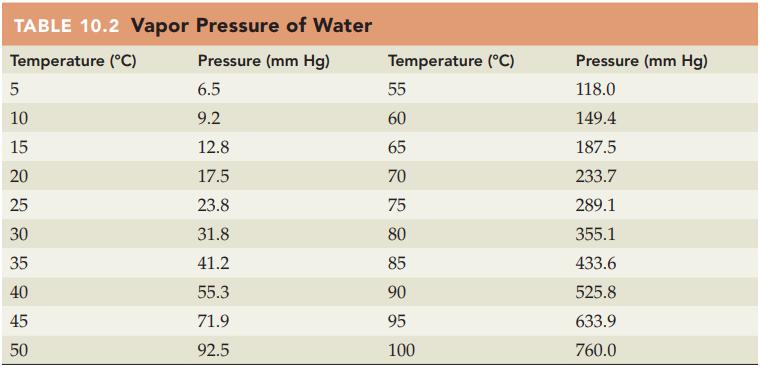
Refer to Table 10.2 and state the vapor pressure for water in mm Hg at each of
Question:
Refer to Table 10.2 and state the vapor pressure for water in mm Hg at each of the following temperatures:
(a) 25 °C
(b) 50 °C.
Table 10.2
Transcribed Image Text:
TABLE 10.2 Vapor Pressure of Water Temperature (°C) Pressure (mm Hg) 6.5 9.2 5 10 15 20 25 30 35 40 45 50 12.8 17.5 23.8 31.8 41.2 55.3 71.9 92.5 Temperature (°C) 55 60 65 70 75 80 85 90 95 100 Pressure (mm Hg) 118.0 149.4 187.5 233.7 289.1 355.1 433.6 525.8 633.9 760.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Temperature C a 25 Co Vapor ...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Table 10.2 and state the vapor pressure for water in atm at each of the following temperatures: (a) 75 C (b) 100 C. Table 10.2 TABLE 10.2 Vapor Pressure of Water Temperature (C) Pressure (mm...
-
You are supplied with a 4.12 % concentration (by weight) hydrogen peroxide solution. Assume the density of your solution is 1.00 g/mL. a. Write a balanced equation for the reaction of aqueous...
-
The HaberBosch process for the production of ammonia is one of the key industrial processes in developed countries. N 2 (g) + 3 H 2 (g) 2 NH 3 (g) (a) Calculate r G for the reaction at 298 K, 800...
-
The following data relating to direct materials cost for March of the current year are taken from the records of Play Tyme Inc., a manufacturer of plastic toys: Quantity of direct materials used...
-
Samsung is a leading global manufacturer that competes with Apple and Google. Key financial figures for Samsung follow. Required 1. What is the return on assets for Samsung in the (a) Current year...
-
Spot price of natural gas. The table shown in the next column lists the spot price of natural gas (in dollars per million Btu) between 2000 and 2020. a. Using 2000 as the base period, calculate and...
-
Julia Dumars is a licensed CPA. During the first month of operations of her business, Julia Dumars, Inc., the following events and transactions occurred. May 1 Stockholders invested $20,000 cash in...
-
Global Value-Price team conducted a price survey from the internet and other information resources. The findings of the survey are gathered in an Excel worksheet, as follows: Item Item Company Item...
-
Which of the following liquids has the higher vapor pressure at 50 C: alcohol or mercury
-
Which of the following liquids has the higher vapor pressure at 25 C: water or mercury?
-
Adjusting Entries: Prepaid Expenses and Unearned Revenues Erickson Group provides computer network consulting services. The company initially debits assets in recording prepaid expenses and credits...
-
The relationship between income and savings, let's look back to the recent credit crisis that sent our economy into the greatest financial crisis since the Great Depression. Watch this short video...
-
Jos Lpez has $15,000 in a 6-year certificate of deposit (CD) that pays a guaranteed annual rate of 4%. Create a timeline showing when the cash flows will occur. (6 points) 2. Oliver Lpez deposits...
-
PROBLEM SET #2 At a large urban college, about half of the students live off campus in various arrangements, and the other half live on campus. Is academic performance dependent on living...
-
Post a compelling argument stating whether leaders are born, made, or a combination of both. Drawing from the discussion of the two current peer-reviewed articles you identified, support your...
-
Unicorn Inc. builds commercial jets and calculate the cost for each jet. For each item below, indicate whether it would be most likely classified as direct labor (DL); direct materials (DM);...
-
Obtain an estimate for the value of e by using Euler's method to approximate the solution of the IVP y' = y, y(0) = 1, at t = 1, using smaller and smaller values of h. As h decreases, the...
-
Which, if any, of the dichloroethene molecules drawn in Data Table II (3.) (4.) and (5.) are geometric isomers? A. B. C. D. cis-1,2-dichloroethene and trans-1,2-dichloroethene...
-
List the six steps in estimating a cost function on the basis of an analysis of a past cost relationship which step is typically the most difficult for the cost analyst?
-
When using the high-low method, should you base the high and low observations on the dependent variable or on the cost driver?
-
Describe three criteria for evaluating cost functions and choosing cost drivers.
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


