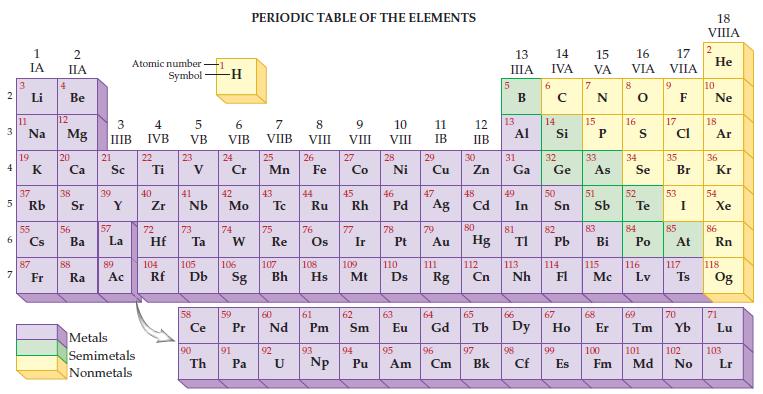
Refer to the periodic table and determine the atomic number and atomic mass for sulfur. 2 3
Question:
Refer to the periodic table and determine the atomic number and atomic mass for sulfur.
Transcribed Image Text:
2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Ac Atomic number Symbol Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta Ce 90 -1 Th -H 6 VIB 24 Cr 42 Mo 74 W 59 91 PERIODIC TABLE OF THE ELEMENTS 7 VIIB Pa 25 Mn 43 75 Re 60 P Pr Nd 92 8 VIII U 26 44 Fe Ru 76 61 Pm 93 9 VIII 104 105 106 107 108 109 110 Rf Db Sg Bh Hs Mt D Np 27 Co 45 Rh 77 Ir 62 Sm 94 10 VIII Pu 28 Ni 46 78 47 Pd Ag 63 11 IB 29 95 Cu 79 Au 111 64 Eu Gd Rg 96 Am Cm 12 IIB 30 Zn 48 Cd 80 Hg 112 Cn 65 97 Bk 13 IIIA 13 31 Ga Al 49 81 In 66 TI 113 Nh 98 Cf 6 14 15 16 IVA VA VIA 14 Si 32 Ge 50 82 Pb 114 67 99 E Es 15 33 As 51 83 Bi 115 Mc 68 8 16 34 Se 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 Cl 35 53 85 Br I At 117 70 Ts 100 101 102 Fm Md No 18 VIIIA 2 He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn 118 Og 71 Lu 103 Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
16 ...View the full answer

Answered By

Sandip Agarwal
I have an experience of over 4 years in tutoring. I have solved more than 2100 assignments and I am comfortable with all levels of writing and referencing.
4.70+
19+ Reviews
29+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and determine the atomic number and mass number listed for element 61, promethium. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB...
-
What would you suggest to be done and which principles are important to you in this decision? How would you think about the greatest good in this case? As a leader what are your duties and who are...
-
The electronics industry manufactures transistors using arsenic diffusion. Refer to the periodic table and predict an element that may substitute for arsenic. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55...
-
Each table of values gives several points that lie on a line.(a) What is the x-intercept of the line? The y-intercept?(b) Which equation in choices AD corresponds to the given table of values?(c)...
-
What are the two dimensions of a total quality management (TQM) program? Why is TQM being used in business practice?
-
Stellar Baking Company in Australia has a trailing P/E of 14. Analysts predict that Stellars dividends will continue to grow at its recent rate of 4.5 percent per year into the indefinite future....
-
Random Number Table Use the seventh row of Table 1 in Appendix B to generate 12 random numbers between 1 and99.
-
The crane shown rotates at the constant rate 1 = 0.25 rad/s; simultaneously, the telescoping boom is being lowered at the constant rate 2 = 0.40 rad/s. Knowing that at the instant shown the length of...
-
Walters Audio Visual, Inc. offers a stock option plan to its regional managers. On January 1, 2018, 60 million options were granted for 60 million $1 par common shares. The exercise price is the...
-
Sodium has only one stable isotope. What is the mass of the Na-23 stable isotope?
-
Refer to the periodic table on the inside cover of this text and determine the atomic number and atomic mass for iron. 2 3 4 5 6 7 3 11 1 IA Li Na 19 37 Rb 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra...
-
An executive's telephone log showed the lengths of 65 calls initiated during the last week of July. (a) Use Excel or MegaStat to sort and standardize the data. (b) Based on the standardized z-scores,...
-
Question 7 Two objects, of masses 3 and 4 kg, are hung from the ends of a stick that is 70 cm long and has marks every 10 cm, as shown above. If the mass of the stick is negligible, at which of the...
-
Since they do not have enough saved, Rachel and John would like to consider retiring later. Create a new timeline and recalculate all of the relevant values to determine at what age Rachel and John...
-
Problem 6 Find the partial derivative with respect to x for the following functions: (a) p = 56 (b) y(x)=56-4x (c) m = r (d) q= x (e) f(x) =x3 (f) g(x,y) = xy 2 (g) h(x,y) = Ax1/2y1/2, where A is a...
-
Consider the information in the file named Cost Functions of the Firm (also presented above). Please read that file carefully before answering this and the following questions. The fixed cost of...
-
On January 1, 2022, Monica Company acquired 80 percent of Young Company's outstanding common stock for $872,000. The fair value of the noncontrolling interest at the acquisition date was $218,000....
-
Give the major product of each of the following reactions: a. b. c. d. ,CH,CH, + HBr CH CH2 CCH2CH3HBr CH CH2 CH CH,CHCCH-CH, + HBr - CH
-
Read the Forecasting Supply Chain Demand Starbucks Corporation case in your text Operations and Supply Chain Management on pages 484-485, then address the four questions associated with the...
-
Assume that it is now July of year 1, and the brothers are developing pro forma financial statements for the following year. Further, assume that sales and collections in the first half-year matched...
-
Assume now that it is several years later. The brothers are concerned about the firm's current credit terms, which are now net 30, which means that contractors buying building products from the firm...
-
Under the current credit policy, what is the firm's days sales outstanding (DSO)? What would the expected DSO be if the credit policy change were made?
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


