What is the mass of 3.36 L of ozone gas, O 3 , at STP? Strategy Plan
Question:
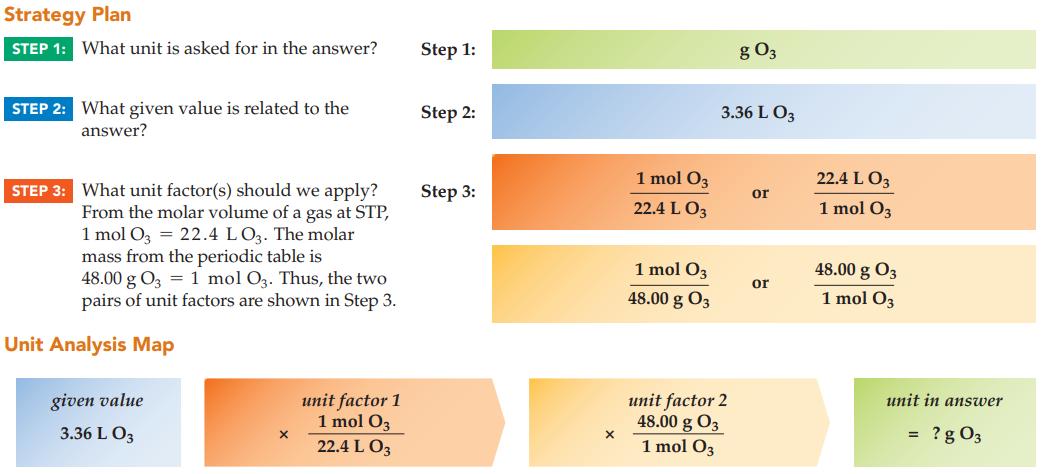
What is the mass of 3.36 L of ozone gas, O3, at STP?
Transcribed Image Text:
Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is related to the answer? STEP 3: What unit factor(s) should we apply? From the molar volume of a gas at STP, 1 mol O3 = 22.4 LO3. The molar mass from the periodic table is 48.00 g 03 1 mol O3. Thus, the two pairs of unit factors are shown in Step 3. Unit Analysis Map given value 3.36 L 03 X unit factor 1 1 mol 03 22.4 L 03 Step 1: Step 2: Step 3: X 1 mol 03 22.4 L 03 1 mol 03 48.00 g 03 g 03 3.36 L 03 unit factor 2 48.00 g 03 1 mol O3 or or 22.4 L 03 1 mol 03 48.00 g 03 1 mol O3 unit in answer ? g 03 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
We apply the unit factor 1 mol 0...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
What are some of the characteristics of the Canadian state that have influenced the way media have developed in Canada? What are some of the ways in which the federal government has played a central...
-
What is the mass of the solid NH4Cl formed when 73.0 g of NH3 are mixed with an equal mass of HCl? What is the volume of the gas remaining, measured at 14.0C and 752 mmHg? What gas is it?
-
What is the mass of solute in 3.81 L of 0.0232 M Zn(NO3)2?
-
An employee earns $24 per hour and 1.5 times that rate for all hours in excess of 40 hours per week. Assume that the employee worked 43 hours during the week. Assume that the FICA tax rate is 7.5%...
-
Presented below are three independent situations. 1. Wakarusa Corporation retired $130,000 face value, 12% bonds on June 30, 2012, at 102. The carrying value of the bonds at the redemption date was...
-
Classify the following as cross-section or time-series data. a. Food bill of a family for each month of 2015 b. Number of accidents each year in Dallas from 2000 to 2015 c. Number of supermarkets in...
-
Analyzing the Buy-versus-Rent Decision. Use the buy-versus-rent analysis to compare two residences you might consider. LO9-2
-
The housekeeping staff at the five-star Ritz Hotel has a specific, detailed list of activities for cleaning an occupied room. Each activity is completed whether or not it looks like it is necessary....
-
Bassman Company operates on a contribution margin of 30% and currently has fixed costs of $400,000. Next year, sales are projected to be $2,000,000. An advertising campaign is being evaluated that...
-
Given that I-127 is the only natural isotope of iodine, what is the mass of one I atom and Avogadros number of I atoms, respectively?
-
What is the volume of one mole of any gas at STP?
-
Hora plc holds inventories of a particular type of motor car tyre, which is ordered in batches of 1,200 units. The supply lead times and usage rates for the tyres are: The business wishes to avoid...
-
For the data in Problem 42, how would you predict demand for medical kits using (a) moving averages and (b) exponential smoothing (with alpha values equal to 0.5 and greater) for the 21st week? Data...
-
For a light ray that crosses the interface between medium 1 having index of refraction \(n_{1}\) and medium 2 having index of refraction \(n_{2}\), what relationship between \(\theta_{1}\) and...
-
The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon dioxide). The carbon dioxide on Venus might be in equilibrium with carbonate ions in minerals on...
-
Seniority quantum numbers typically measure how many fermions are in some sense "not paired" with another fermion. For the quasispin model of Problem 31.3 , define the Racah seniority $v$ through...
-
(a) Place a perfectly conducting sphere with radius a in a uniform electric field E 0 and let an origin centered electric dipole field represent the field produced by the sphere. Use this information...
-
Use the recursion relation (5.98) and normalization to find (a) Y03; (b) Y13.
-
How do network effects help Facebook fend off smaller social-networking rivals? Could an online retailer doing half as much business compete on an equal footing with Amazon in terms of costs? Explain.
-
Lower-of-Cost-or-Market Remmers Company manufactures desks. Most of the company's desks are standard models and are sold on the basis of catalog prices. At December 31, 2010, the following finished...
-
Lower-of-Cost-or-Market Garcia Home Improvement Company installs replacement siding, windows, and louvered glass doors for single family homes and condominium complexes in northern New Jersey and...
-
Entries for Lower-of-Cost-or-Market'Direct and Allowance Malone Company determined its ending inventory at cost and at lower-of-cost-or-market at December 31, 2009, December 31, 2010, and December...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


