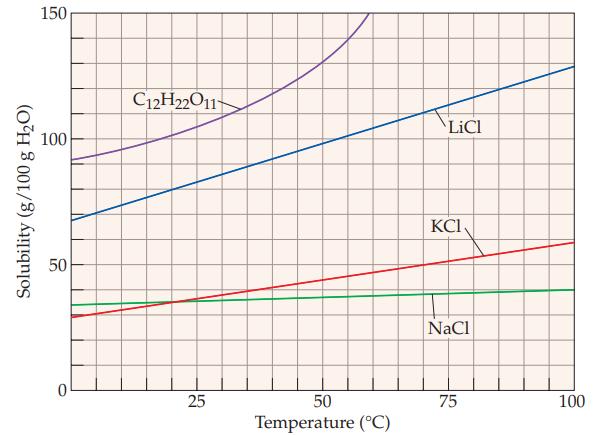
What is the solubility of C 12 H 22 O 11 at 20 C? (Refer to Figure
Question:
What is the solubility of C12H22O11 at 20 °C? (Refer to Figure 13.5)
(a) 20 g/100 g water
(b) 55 g/100 g water
(c) 100 g/100 g water
(d) 140 g/100 g water
(e) 150 g/100 g water.
Figure 13.5
Transcribed Image Text:
Solubility (g/100 g H₂O) 150 100 50 C12H22O11 25 50 Temperature (°C) LiCl KCI NaCl 75 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The solubility of C12H22O11 at 20 C is c 100 g100 g water The graph you referred to shows the so...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The solubility of carbon dioxide in water is 0.161 g CO2 in 100 mL of water at 20C and 1.00 atm. A soft drink is carbonated with carbon dioxide gas at 5.50 atm pressure. What is the solubility of...
-
Star Company has the following sales, variable cost, and fixed cost. If sales increase by $10,000 then their profit increases/decreases by how much? Sales $50,000 Variable...
-
When does self control begin, and how does it change as children's develop?
-
What is the potential difference across one wire of a 30-m extension cord made of 16-gauge copper wire carrying a current of 5.0 A?
-
If a parent uses the equity method but does not amortize the difference between fair value and book value on its separate books, its net income and retained earnings will not equal its share of...
-
A small manufacturing company makes only credit sales. If cash receipts from sales are misappropriated, which of the following acts would most likely conceal this fraud? a. Understating the accounts...
-
Define the term predetermined and standard cost.
-
1. What did Arthur Andersen contribute to the Enron disaster? 2. What Arthur Andersen decisions were faulty? 3. What was the prime motivation behind the decisions of Arthur Andersens audit partners...
-
Materials used by the Instrument Division of T _ Kong Industries are currently purchased from outside suppliers at a cost of $ 3 6 0 per unit. However, the same materials are available from the...
-
Refer to the solubility behavior shown in Figure 13.5 and determine the minimum temperature required to obtain the following solutions. (a) 35 g NaCl per 100 g of water (b) 45 g KCl per 100 g of...
-
Given a hot cup of coffee, and a warm cup of coffee, which can dissolve more sugar?
-
Show that the circuit in Figure P16.82 is a J-K flip-flop. CLK o Figure P16.82 VDD D Sp CLK RD
-
How have you maintained your medical billing skills over the past 12 months? Include any courses or learning opportunity you used to build your current knowledge base. How did these skills help you?...
-
1. What issues does Bob Holland face as he takes over as CEO of Ben & Jerry's? Which are the most important? 2. Where is the market headed? What are the competitive influences and compare the...
-
Do you think there is a difference between diversity management and affirmative action? Provide an explanation for your response. Support your response with APA cited references. Response: Diversity...
-
1. In what ways do practical and statistical significance work together to help us understand program effects? Can one be important to aprogram evaluator withoutthe other? If so, how? If not, why...
-
How do IT metrics, measurements, productivity, and efficiency work together? Make sure you explain each word.Make sure to pick out two or three specific IT data and measures. Also, back up what you...
-
The general ledger of the Fly-Buy-Nite (FBN) Engineering Company contained the following account balances (partial listing) at the end of June, 2004. Construct an income statement. What is the net...
-
Currently, there are five concepts of food stands, including: hot dogs, soft pretzels, turkey legs, sandwich wraps, and funnel cakes. This approach will double the existing number of food stands...
-
Using Return Distributions assuming that the returns from holding small company stocks are normally distributed, what is the approximate probability that your money will double in value in a single...
-
Distributions in Problem 18, what is the probability that the return is less than100 percent (think)? What arc the implications for the distribution of returns?
-
Blumes Formula Over a 30-year period an asset had an arithmetic return of 12.8 percent and a geometric return of 10.7 percent. Using Blumes formula, what is your best estimate of the future annual...
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


