Without referring to Table 11.6, predict which compound in each of the following pairs has the higher
Question:
Without referring to Table 11.6, predict which compound in each of the following pairs has the higher heat of fusion (cal/mol):
(a) H2O or H2S
(b) H2S or H2Se.
Table 11.6
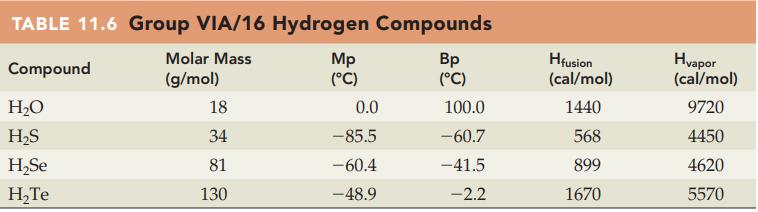
Transcribed Image Text:
TABLE 11.6 Group VIA/16 Hydrogen Compounds Molar Mass Compound (g/mol) H₂O H₂S H₂Se H₂Te 18 34 81 130 Mp (°C) 0.0 -85.5 -60.4 -48.9 Bp (°C) 100.0 -60.7 -41.5 -2.2 Hfusion (cal/mol) 1440 568 899 1670 Hvapor (cal/mol) 9720 4450 4620 5570
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Predicting Heat of Fusion Heres how we can predict the compound with the higher heat of fusion in ea...View the full answer

Answered By

Caroline Kinuthia
Taking care of the smaller details in life has a larger impact in our general well being, and that is what i believe in. My name is Carol. Writing is my passion. To me, doing a task is one thing, and delivering results from the task is another thing. I am a perfectionist who always take things seriously and deliver to the best of my knowledge.
4.90+
1934+ Reviews
4278+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Which compound in each of the following pairs has the higher boiling point? (Answer this problem without consulting tables.) (a) Pentanal or 1-pentanol (b) 2-Pentanone or 2-pentanol (c) Pentane or...
-
Which compound in each of the following pairs would have the higher boiling point? Explain your answers. (a) (b) (c) (d) (e) (f) (g) (h) Hexane, CH3(CH2)4CH3, or nonane, CH3(CH2)7CH3 (i) OH or HO OH...
-
Which compound in each of the following pairs is a stronger base? Why? a. b. or NH NH CH3CHCH or CH CNH2
-
The _____________ works out the best way to structure finances and make effective financial decisions.
-
On July 1, 2012, Kim Wheeler established an interior decorating business, Aztec Designs. During the month, Kim completed the following transactions related to the business: July 1. Kim transferred...
-
Select one of the two choices to complete the following sentence, and then explain your choice: The absence of certainty about the health effects of an environmental pollutant (is/is not) synonymous...
-
Using the data in problem 8.12 and the seasonal indices you have calculated, calculate expected monthly demand if the annual forecast is 2000 units. Month Seasonal Index Forecast January February...
-
Phil Jackson, after winning his tenth NBA title as a coach, said: I dont motivate my players. You cannot motivate someone. All you can do is provide a motivating environment and the players will...
-
In order of most liquid Prepare trial balance. P3.10B (LO.5) You are presented with the following alphabetical list of accounts and balances (in thousands) for Asian Importers Limited at January 31,...
-
Without referring to Table 11.6, predict which compound in each of the following pairs has the higher heat of vaporization (cal/mol): (a) H 2 O or H 2 Se (b) H 2 S or H 2 Te. Table 11.6 TABLE 11.6...
-
A solid cube of ammonia floats in liquid ammonia. Which is more dense: solid or liquid ammonia?
-
For a two-band perfect reconstruction filter bank, assume the analysis filter \(H_{0}(z)\) and the synthesis filter \(G_{0}(z)\) satisfy the condition of Equation (10.142). Assume also that these...
-
1. create a concept map for 0D, 1D, 2D and 3D crystals 2. write down the formulas for quantifying numbers of defects
-
\fNOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF AMERICAN AIRLINES GROUP INC . Commitments , Contingencies and Guarantees ( 2 ) Aircraft and Engine Purchase Commitment Under all of our aircraft and...
-
Critical Values. In Exercises 41-44, find the indicated critical value. Round results to two decimal places. 41. Z0.25 42. Z0.90 43. Z0.02 44. 20.05
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
In todays social and business environments, some organizations only talk the talk regarding ethics and ethical conduct rather than walk the ethical organizational path. In what ways can ethical and...
-
Consider the following alternatives: Use present worth analysis, an 8% interest rate, and an infinite analysis period. Which alternative should be selected in each of the two following situations? 1....
-
Perform the operation by first converting the numerator and denominator to scientific notation. Write the answer in scientific notation. 7,200,00/0.000009
-
Refer again to the Circular File balance sheet in Section 20.2. Suppose that the government suddenly offers to guarantee the $50 principal payment due bondholders next year and also to guarantee the...
-
Is it more valuable to own an option to buy a portfolio of stocks or to own a portfolio of options to buy each of the individual stocks? Say briefly why.
-
Table 20.4 lists some prices of options on common stocks (prices are quoted to the nearest dollar). The interest rate is 10 percent a year. Can you spot any mispricing? What would you do to take...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


