Without referring to Table 11.6, predict which compound in each of the following pairs has the higher
Question:
Without referring to Table 11.6, predict which compound in each of the following pairs has the higher melting point:
(a) H2O or H2S
(b) H2S or H2Se.
Table 11.6
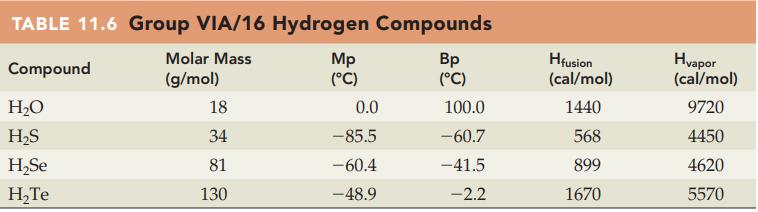
Transcribed Image Text:
TABLE 11.6 Group VIA/16 Hydrogen Compounds Molar Mass Compound (g/mol) H₂O H₂S H₂Se H₂Te 18 34 81 130 Mp (°C) 0.0 -85.5 -60.4 -48.9 Bp (°C) 100.0 -60.7 -41.5 -2.2 Hfusion (cal/mol) 1440 568 899 1670 Hvapor (cal/mol) 9720 4450 4620 5570
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Predicting the melting point of a compoundwithout referring to a tablerequires considering the inter...View the full answer

Answered By

Rohith Bellamkonda
I am studying in IIT Indore,the most prestigious institute of India.I love solving maths and enjoy coding
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Which compound in each of the following pairs has the higher boiling point? (Answer this problem without consulting tables.) (a) Pentanal or 1-pentanol (b) 2-Pentanone or 2-pentanol (c) Pentane or...
-
Which compound in each of the following pairs would have the higher boiling point? Explain your answers. (a) (b) (c) (d) (e) (f) (g) (h) Hexane, CH3(CH2)4CH3, or nonane, CH3(CH2)7CH3 (i) OH or HO OH...
-
Which compound in each of the following pairs is a stronger base? Why? a. b. or NH NH CH3CHCH or CH CNH2
-
Posting the entries from the sales journal to the accounts receivable subsidiary ledger should be done ______. a). at the end of each month b). on a weekly basis c). on a daily basis d). only at the...
-
Maui Outfitters Corporation manufactures and distributes leisure clothing. Selected transactions completed by Maui Outfitters during the current fiscal year are as follows: Feb 19. Split the common...
-
Do you think this cartoon reasonably depicts the challenge of reducing pesticide use? OUke Baldwin / Correret BAA ORGANIC * NO PESTICIDES MARKET $25 425. 6 18 "Sure it costs more. We have to squash...
-
Given the following forecast and actual demand, calculate the mean absolute deviation. Period Forecast Actual Demand Absolute Deviation 1 110 85 2 110 105 3 110 120 4 110 100 5 110 90 Total LO.1
-
On March 4, 2016, Hein Corporation issues 1,000 shares of $100 par preferred stock LO 15.6 for $125 per share. The stock is not callable by the corporation until 3 years have expired. On April 7,...
-
Cap Incorporated manufactures ball point pens that sell at wholesale for $0.80 per unit. Budgeted production in both 2021 and 2022 was 14,000 units. There was no beginning inventory in 2021. The...
-
Which liquid has the highest heat of fusion and heat of vaporization?
-
Which liquid has the highest specific heat?
-
Determine the simple interest. Unless noted otherwise, assume the rate is an annual rate. Assume 360 days in a year. Round answers to the nearest cent. p = $2500, r = , t = 9 months 3 %
-
1. Using appropriate examples, compare and contrast the genetic diversity of marine fish species with freshwater fish species (8 marks) 2. Your class went on a trip and discovered a crater lake on...
-
Find sin(29) given that cos(0) = and 0
-
Amazing Aquariums began as a class project on new business development. Now that the visionaries behind the idea have graduated, they want to explore their business idea and see if the concept could...
-
3. Modify the program of Example 05 so that, it takes and prints values using the following two functions respectively. void get (double *&a, int& n); void print (double *a, int n); 4. Following is a...
-
A company will be financing its operations with and a capital budget is P40,000,000 and a debt-to-equity ratio of 1. The interest rate on company's debt is 10%. The expected return on equity by the...
-
A firm is considering two alternatives: At the end of 4 years, another B may be purchased with the same cost, benefits, and so forth. If the MARR is 10%, which alternative should be selected? $10,700...
-
Determine two different Hamilton circuits in each of the following graphs. A B F G
-
Recalculate the value of the Buffelhead call option (see question 5), assuming that the option is American and that at the end of the first six months the company pays a dividend of $25. (Thus the...
-
Suppose that you have an option which allows you to sell Buffelhead stock (see question 5) in month 6 for $165 or to buy it in month 12 for $165. What is the value of this unusual option?
-
The current price of the stock of Mont Tremblant Air is C$100. During each six-month period it will either rise by 11.1 percent or fall by 10 percent (equivalent to an annual standard deviation of...
-
Discuss American History
-
Your firm has developed a new lithium ion battery polymer that could enhance the performance of lithion ion batteries. These batteries have applications in many markets including cellphones, laptops,...
-
Need help analyzing statistical data 1. ANOVA) True or false: If we assume a 95% confidence level, there is a significant difference in performance generally across all groups. 2. (t-test) True or...

Study smarter with the SolutionInn App


