Write a balanced equation for each of the following redox reactions in an acidic solution using the
Question:
Write a balanced equation for each of the following redox reactions in an acidic solution using the half-reaction method.
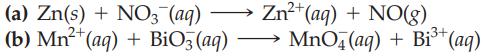
Transcribed Image Text:
(a) Zn(s) + NO3- (aq) (b) Mn²+ (aq) + BiO3(aq) Zn²+ (aq) + NO(g) MnO4 (aq) + Bi³+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a 3 Zns 2 NO 3 aq 8 H aq ...View the full answer

Answered By

ZIPPORAH KISIO LUNGI
I have worked on several other sites for more than five years, and I always handle clients work with due diligence and professionalism. Am versed with adequate experience in the fields mentioned above in which have delivered quality papers in research, thesis, essays, blog articles, and so forth.
I have gained extensive experience in assisting students to acquire top grades in biological, business and IT papers. Notwithstanding that, I have 7+ years of experience in corporate world software design and development.
5.00+
194+ Reviews
341+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced equation for each of the following redox reactions in a basic solution using the half-reaction method. (a) MnO4 (aq) + S(aq) (b) Cu(s) + CIO (aq) MnO(s) + S(s) Cu+ (aq) + Cl (aq)
-
Write a balanced equation for each of the following reactions or reaction sequences. (a) The reaction catalyzed by PFK-2 (b) The conversion of 2 moles of oxaloacetate to glucose (c) The conversion of...
-
Write a balanced equation for each of the following redox reactions using the oxidation number method. (a) Fe 2+ (aq) + H 2 O 2 (aq) + H + (aq) Fe 3+ (aq) + H 2 O(l) (b) Cr 2 O 7 2 (aq) + Br (aq) +...
-
In recent years, Avery Transportation purchased three used buses. Because of frequent turnover in the accounting department, a different accountant was in charge of selecting the depreciation method...
-
Prepare journal entries to record these transactions: (a) Benton Company retires its delivery equipment, which cost $41,000. Accumulated depreciation is also $41,000 on this delivery equipment. No...
-
What measures do we use for the size of small entities in memory, such as ints and strings?
-
2. Assuming a $10m investment in one stock, compute the 95% and 99% VaR for stocks A and B over 1-day, 10-day, and 20-day horizons. assume that the risk-free rate is 0.08 and that there are three...
-
In a recent year, Coach, Inc, a designer and marketer of handbags and other accessories, issued 12,100 shares of its $0.01 par value stock for $344,000 (these numbers are rounded). These additional...
-
PEM, Incorporated, is experiencing financial difficulty due to erratic sales of its only product, a high - capacity battery for laptop computers. The company s contribution format income statement...
-
Write a balanced half-reaction for each of the following in a basic solution. (a) Ni(OH) 2 (s) NiO 2 (s) (b) NO 2 (aq) N 2 O(g).
-
Write a balanced half-reaction for each of the following in an acidic solution. (a) H 2 O 2 (aq) H 2 O(l) (b) AsO 3 3 (aq) AsO 3 (aq).
-
Firm X has the opportunity to invest $200,000 in a new venture. The projected cash flows from the venture are as follows. Firm X uses an 8 percent discount rate to compute NPV, and its marginal tax...
-
CLT HW Score: 0/19 0/19 answered Question 4 < = 31. You intend to draw a A population of values has a normal distribution with = 232.9 and random sample of size n = 165. Please show your answers as...
-
Q-3: Estimate fxy dx + x2 dy: where c is given by [Hint: Use Green's theorem -1
-
1. Determine completely the resultant of the four forces shown in the figure. Each force makes a 15 angle with the vertical, except the 200 N force which is vertical. Find the action line (position)...
-
1: Based on the results of your Learning Style produce a 1 pg reflection. (this is the result of the test i took: Your learning preference:Multimodal (AK) SharePeople with your preference like:...
-
Explain what the petty cashier should do if he or she thinks that the imprest amount is inadequate.
-
An arithmetic sequence of six numbers begins with 7 and ends with 27. Follow 11a-c to find the four missing terms. a. Name two points on the graph of this sequence: (?, 7) and (?, 27). b. Plot the...
-
How can NAFTA be beneficial to suppliers of Walmart?
-
The following accounts and their balances were selected from the unadjusted trial balance of REO Inc., a freight forwarder, at October 31, the end of the current fiscal year: Preferred 2% Stock, $100...
-
The following accounts and their balances appear in the ledger of Newberry Properties Inc. on June 30 of the current year: Common Stock , $75 par $1,350,000 Paid-In Capital in Excess of Par 108,000...
-
Race Car Inc. retails racing products for BMWs, Porsches, and Ferraris. The following accounts and their balances appear in the ledger of Race Car Inc. on April 30, the end of the current year:...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


