In the cryoscopic determination of molar mass, the molar mass in kg mol 1 is given by
Question:
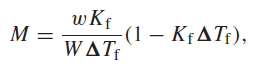
where W is the mass of the solvent, w is the mass of the unknown solute, ΔTf is the amount by which the freezing point of the solution is less than that of the pure solvent, and Kf and Kf are constants characteristic of the solvent. A sample of an unknown substance was dissolved in benzene, for which Kf = 5.12 K kg molˆ’1 and Kf = 0.011 Kˆ’1. For the following data, calculate M and its probable error:
W = 13.185 ± 0.003 g,
w = 0.423 ± 0.002 g,
ΔTf = 1.263 ± 0.020 K.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





