The vibrational contribution to the molar heat capacity of a gas of nonlinear molecules is given in
Question:
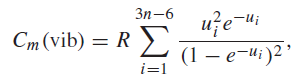 i=1 " style="" class="fr-fic fr-dib">
i=1 " style="" class="fr-fic fr-dib">
where ui = hνi/kBT . Here νi is the frequency of the ith normal mode of vibration, of which there are 3n ˆ’ 6 if n is the number of nuclei in the molecule, h is Planck€™s constant, kB is Boltzmann€™s constant, R is the ideal gas constant, and T is the absolute temperature. The H2O molecule has three normal modes. The frequencies are given by
ν1 = 4.78 × 1013 sˆ’1 ± 0.003 × 1013 sˆ’1,
ν2 = 1.095 × 1014 sˆ’1 ± 0.004 × 1014 sˆ’1,
ν3 = 1.126 × 1014 sˆ’1 ± 0.005 × 1014 sˆ’1.
Calculate the vibrational contribution to the heat capacity of H2O vapor at 500.0 K and find the 95% confidence interval.Assume the temperature to be fixed without error.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





