Find the damping parameters and natural frequencies of the systems governed by the following second-order linear constant-coefficient
Question:
Find the damping parameters and natural frequencies of the systems governed by the following second-order linear constant-coefficient differential equations:
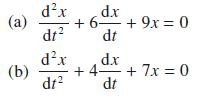
Transcribed Image Text:
(a) (b) dx dt dx dt dx + 6- + 9x=0 dt d.x +4- + 7x=0 dt
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
In each part of this question we compare the se...View the full answer

Answered By

Navashree Ghosh
I believe in quality work and customer satisfaction. So, I can assure you that you will get quality work from me when you hire me. Let's work together and build a long-term association.
4.90+
82+ Reviews
116+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Find the damping parameters and natural frequencies of the systems governed by the following second-order linear constant-coefficient differential equations: (a) dx dt (b) 2- (d) dx dt d.x + 2a- dt...
-
Determine the values of the appropriate parameters needed to give the systems governed by the following second-order linear constant-coefficient differential equations the damping parameters and...
-
Determine the values of the appropriate parameters needed to give the systems governed by thefollowing second-order linear constant-coefficient differential equations the damping parameters and...
-
The number of letter misprints per page of a book, where 24 pages have been taken at random from this book, is given below. Draw and appropriate control chart and provide interpretation. Page 1 2...
-
An electron is released from rest and falls under the influence of gravity. In the first centimeter, what fraction of the potential energy lost is radiated away?
-
Operating the channel does not imply total control of the channel. Can you think of an example where the channel manager does not have total control of the channel but is still able to operate it?
-
Prepare Hertog Companys journal entries to reflect the following transactions for the current year. May 7 Purchases 200 shares of Kraft stock as a short-term investment in available-for-sale...
-
When Kraft recently bid $16.7 billion for Cadbury, Cadbury's market value rose, but Kraft's market value fell by more. What does this tell you about the value-creating potential of the deal?
-
On January 1, a company issues bonds dated January 1 with a par value of $370,000. The bonds mature in 5 years. The contract rate is 11%, and interest is paid semiannually on June 30 and December 31....
-
The function A() is as given by (10.55) and shown in Figure 10.27. Show that A() has a simple maximum point when max. Find max as a function of and , and also find A( max ). For >, A(2) has no...
-
Show that the characteristic equation of the differential equation and hence find the general solutions of the equations is dx dt dx 9- df +27x - 27x=0 dt (m3) = 0
-
Discuss the extent to which developments in audit automation are helping further to fracture the auditing profession into two distinct groups: a small number of firms which audit almost all the large...
-
Gulf Shore Lawn and Garden Maintenance provides two general outdoor services: lawn maintenance and garden maintenance. The company charges customers $18.0 per hour for each type of service, but lawn...
-
Two level sections of an east highway (G=0) are to be connected. Currently, the two sections of highway are seperated by a 4000-ft (horizontal distance), 2% grade. The westernmost section of highway...
-
A solution contains 2 x 10-3 moles Ca2+/L and 3 x 10-4 moles Mg2+/L. Given the formation constants for CaEDTA2- and MgEDTA2- of 1010.6 and 108.7, respecively, calculate: 1) Concentration of MgEDTA2-...
-
The direct material (DM) price variance is $2,650 favorable and the DM usage variance is $3,000 unfavorable. The budgeted amount of DM for each unit of product is 2 lbs. to be purchased at the...
-
On January 1, 2023, AMI Corporation purchased the non-cash net assets of Oriole Ltd. for $8,399,900. Following is the statement of financial position of Oriole Ltd. from the company's year- end the...
-
Air at 1 atm, 13oC and 55 percent relative humidity is first heated to 28oC in a heating section and then is passed through an evaporative cooler where its temperature drops to 22oC. Determine (a)...
-
You are a Loan Officer with an Investment Bank. Today you need to set your lending parameters. They are: LTV: 55% 10 Year T-Bill: TBD Rate Markup: 300 Basis Points Term: 30 Years Amortization: 30...
-
Show the tripeptide that result from linking the amino acids glycine, valine, and serine in that order. Label the N-terminus and C-terminus ends of the tripeptide. | N - -- HN --- -- HN-C-- ...
-
Predict the silicate structure for the mineral talc Mg 3 Si 4 O 10 (OH) 2 that serves as the basis for talcum powder, and show that the formula is charge neutral.
-
Calculate the oxidation state for Cl in the compounds: a) Cl 2 O 6 b) ClF 3 c) HClO 4
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


