Question: (a) Compute e -a from Equation 8-44 for O 2 gas at standard conditions. (b) At what temperature is e -a = 1 for O
(a) Compute e-a from Equation 8-44 for O2 gas at standard conditions.
(b) At what temperature is e-a = 1 for O2?
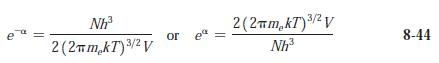
e Nh 2 (2mkT) / V 3/2 or e 3/2 2(2mkT) / V Nh 8-44
Step by Step Solution
3.40 Rating (172 Votes )
There are 3 Steps involved in it
a b a 0 N V hc h 2 MkT 3 NA V... View full answer

Get step-by-step solutions from verified subject matter experts


