Question: Lithium, beryllium, and boron (Z = 3, 4, and 5, respectively) have very low abundances in the cosmos compared to many heavier elements (see Figure
Lithium, beryllium, and boron (Z = 3, 4, and 5, respectively) have very low abundances in the cosmos compared to many heavier elements (see Figure 13-33). Considering the fusion of He to C, explain these low abundances.
Figure 13-33
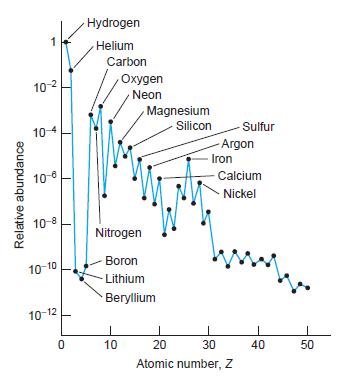
Relative abundance 1 10- 10-4 10-8 10-8 10-10 10-12 0 Hydrogen Helium Carbon Oxygen Neon 1 10 Magnesium Nitrogen Boron Lithium Beryllium Silicon -Sulfur Argon Iron Calcium Nickel 1 20 30 Atomic number, Z 40 50
Step by Step Solution
3.33 Rating (159 Votes )
There are 3 Steps involved in it
The fusion of 1 H to 4 He proceeds via the protonproton ... View full answer

Get step-by-step solutions from verified subject matter experts


