Singly ionized helium He + is hydrogenlike. (a) Construct a carefully scaled energy-level diagram for He +
Question:
Singly ionized helium He+ is hydrogenlike.
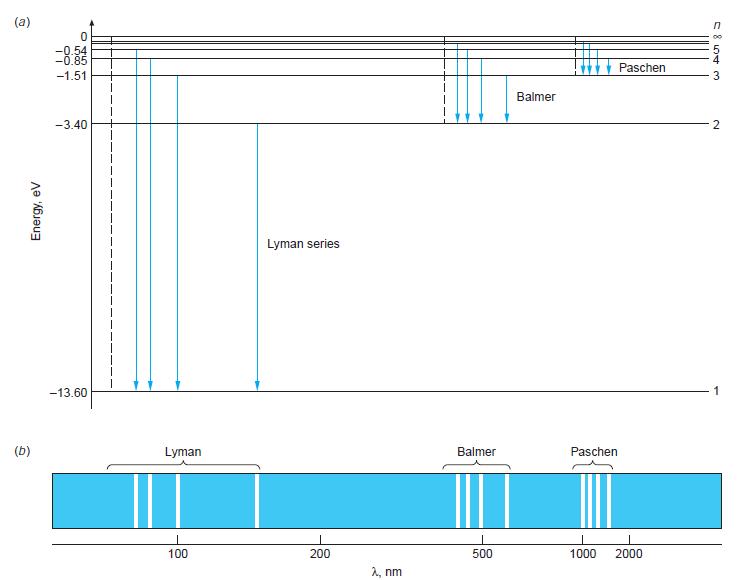
(a) Construct a carefully scaled energy-level diagram for He+ similar to that in Figure 4-16, showing the levels for n = 1, 2, 3, 4, 5, and ∞.
(b) What is the ionization energy of He+?
(c) Compute the difference in wavelength between each of the first two lines of the Lyman series of hydrogen and the first two lines of the He+ Balmer series. Be sure to include the reduced mass correction for both atoms.
(d) Show that for every spectral line of hydrogen, He+ has a spectral line of very nearly the same wavelength. (Mass of He+= 6.65 x 10-27 kg.)
Figure 4-16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





