Question: The molar heat capacity data given in Table 8-2 are taken from AIP Handbook, 2d ed. (McGraw-Hill, New York, 1963). Plot the data for these
The molar heat capacity data given in Table 8-2 are taken from AIP Handbook, 2d ed. (McGraw-Hill, New York, 1963). Plot the data for these solids all on one graph and sketch in the curves CV versus T. Estimate the Einstein temperature for each of the solids using the result of Problem 8-31.
Problem 8-31
Use Equation 8-62 to calculate the value of CV for a solid at the Einstein temperature TE = hf/k.
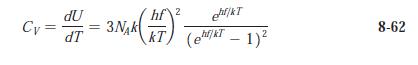
Table 8-2
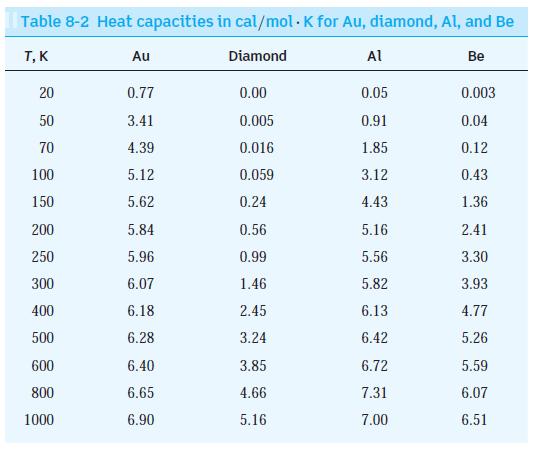
du Cy= = dT 2 3NAK 3 k (17) - ehf/kT (ehf/kT - 1) 8-62
Step by Step Solution
3.48 Rating (164 Votes )
There are 3 Steps involved in it
From the graph T E Au 136K T E Al 243... View full answer

Get step-by-step solutions from verified subject matter experts


