The value of the constant C 200 in Equation 7-33 is Find the values of (a) ,
Question:
The value of the constant C200 in Equation 7-33 is
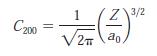
Find the values of
(a) Ψ,
(b) Ψ2, and
(c) The radial probability density P(r) at r = a0 for the state n = 2, ℓ = 0, m = 0 in hydrogen. Give your answers in terms of a0.
Transcribed Image Text:
C₂00 Z3/2 1 √2a0.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
a b c 200 1 ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
The value of Henrys constant for CO2 gas dissolved in water at 290 K is 12.8 MPa. Consider water exposed to atmospheric air at 100 kPa that contains 3 percent CO2 by volume. Under phase equilibrium...
-
In each case, determine the value of the constant c that makes the probability statement correct. a. (c)= .9838 b. P(0 Z c) = .291 c. P(c Z) = .121 d. P(-c Z c) = .668 e. P(c |Z|) = .016
-
The value of the constant C 2,0,0 in Equation 37-36 is Find the values of (a) , (b) 2 , and (c) The radial probability density P(r) at r = a 0 for the state n = 2, = 0, m = 0 in hydrogen. Give your...
-
A sales invoice included the following information: merchandise price, $12,000; terms 1/10, n/eom, FOB shipping point with prepaid freight of $900 added to the invoice. Assuming that a credit for...
-
Betram Chemicals Company processes a number of chemical compounds used in producing industrial cleaning products. One compound is decomposed into two chemicals: anderine and dofinol. The cost of...
-
Evaluate the success of a training program.
-
Quantum tunneling. At temperatures approaching absolute zero 1 -273C2, helium exhibits traits that seem to defy many laws of Newtonian physics. An experiment has been conducted with helium in solid...
-
Based on the most recent competitive threats, what do you predict for Nokia in the coming years? What brand of cell phone do you own? If youre living in the United States, chances are it isnt a...
-
= Homework: Chapter 7 Homework Question 1, E7-14 (book Pan 2012 HW Score: 4,67%, 0.7 of 15 points Points: 0,7 of 2 Save Review the following transactions Click the icon to wow the transactions)...
-
Pathfinder College is a small liberal arts college that wants to improve its admissions process. In particular, too many of its incoming freshmen have failed to graduate for a variety of reasons,...
-
Show that an electron in the n = 2, = 1 state of hydrogen is most likely to be found at r = 4a 0 .
-
If a classical system does not have a constant charge-to-mass ratio throughout the system, the magnetic moment can be written where Q is the total charge, M is the total mass, and g 1. (a) Show that...
-
The Bailey Company has had a defined benefit pension plan for several years. At the end of 2007 the companys actuary provided the following information for 2007 regarding the pension plan: (1)...
-
Gary Tuttle has Citiwide Insurance with 100% coverage after a $25.00 copay on office visits. His services today include an office visit ($62.00), urinalysis with differential ($65.00) and a Treadmill...
-
The Elgin Golf Dutton Golf Merger Elgin Golf Inc. has been in merger talks with Dutton Golf Company for the past six months. After several rounds of negotiations, the offer under discussion is a...
-
f ( x ) = x ^ 3 - 3 x ^ 2 - 2 4 x + 5 6 find all critical numbers
-
Suppose a beam of electrons is aimed at two slits in a slide placed in front of a screen. After a short time, the screen looks like the one at the right. a. What evidence does the picture give that...
-
On January 1, Mitzu Company pays a lump-sum amount of $2,700,000 for land, Building 1, Building 2, and Land Improvements 1. Building 1 has no value and will be demolished. Building 2 will be an...
-
Hanging out. (a) A block of mass m = 20 kg hangs from two cables attached to a ceiling as shown in Figure P4.89A. What is the approximate tension in each cable? (b) The two cables are now rearranged...
-
Imagine a sound wave with a frequency of 1.10 kHz propagating with a speed of 330 m/s. Determine the phase difference in radians between any two points on the wave separated by 10.0 cm.
-
Are the following statements true or false? Explain your answer. (a) The magnitude of a vector can be different in different coordinate systems. (b) The direction of a vector can be different in...
-
A runner is training for an upcoming marathon by running around a 100-m-diameter circular track at constant speed. Let a coordinate system have its origin at the center of the circle with the x-axis...
-
A runner is training for an upcoming marathon by running around a 100-m-diameter circular track at constant speed. Let a coordinate system have its origin at the center of the circle with the x-axis...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


