Work out the quadratic Zeeman effect for the ground-state hydrogen atom [(x|0) = (1/a 0 3 )
Question:
Work out the quadratic Zeeman effect for the ground-state hydrogen atom [(x|0) = (1/√πa03) e-r/a0] due to the usually neglected e2A2/2mec2- -term in the Hamiltonian taken to first order. Write the energy shift as![]()
and obtain an expression for diamagnetic susceptibility, X. The following definite integral may be useful: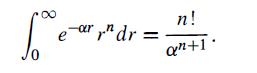
Transcribed Image Text:
A=-1/XB²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
The quadratic Zeeman effect refers to the splitting of atomic energy levels in the presence of a mag...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The hydrogen atom is composed of one proton in the nucleus and one electron, which moves about the nucleus. In the quantum theory of atomic structure, it is assumed that the electron does not move in...
-
Consider Devine Fashion from S8-6. Assume that the fixed expenses assigned to each department include only direct fixed costs of the department (rather than unavoidable fixed costs as given in S8-6):...
-
Explain the technique known as destructive update.
-
Devise a rule which tells how to turn an atom into an anion. Devise a rule which tells how to turn an atom into a cation. Both rules must include subrules which determine what the charge of the ion...
-
Identify at least eight milestones for the Recreation and Wellness Intranet Project. Write a short paper describing each milestone using the SMART criteria. Discuss how determining these milestones...
-
Which of the arguments for tariffs do you feel are most relevant in todays world?
-
corect. Chapter Selerences Book &Resources MacBook Pro corect. Chapter Selerences Book &Resources MacBook Pro
-
Suppose the electron had a very small intrinsic electric dipole moment analogous to the spin-magnetic moment (that is, el proportional to ). Treating the hypothetical - el . E interaction as a small...
-
Work out the Stark effect to lowest nonvanishing order for the n = 3 level of the hydrogen atom. Ignoring the spin-orbit force and relativistic correction (Lamb shift), obtain not only the energy...
-
Why are problem solving and decision making important in sports?
-
In countries with high unemployment and poverty rates, the nation's people are often more concerned with the economic environment than the intricacies of its political systems. In 2010, Mohamed...
-
Analyze this approach: Consider that you could increase the productivity of your department, you have thought about certain ways to do it, but you are not sure. Your team has a lot of experience, but...
-
What is the research question or objective? What research methods did the authors use? Examples include survey, case study, interviews, opinions, qualitative, quantitative, etc. What are the...
-
Provide a critical reflection on each department outlining which services, aspects and operational factors you should further investigate to help improve customer satisfaction. Express the negative...
-
1. Many courses use group projects. What are some of the things that make positive group project experiences? 2. How can a manager motivate employees? Give some specific ideas. Include when you've...
-
Accorsi & Sons specializes in selling and installing upscale home theater systems. On March 1, 2016, Accorsi sold a premium home theater package that includes a projector, set of surround speakers,...
-
Representative data read from a plot that appeared in the paper Effect of Cattle Treading on Erosion from Hill Pasture: Modeling Concepts and Analysis of Rainfall Simulator Data (Australian Journal...
-
What is the formal concentration (expressed as mol/L = M) of NaCl when 32.0 g are dissolved in water and diluted to 0.500 L?
-
How many grams of boric acid, B(OH) 3 (FM 61.83), should be used to make 2.00 L of 0.050 0 M solution? What kind of flask is used to prepare this solution?
-
Describe how you would prepare approximately 2 L of 0.050 0 m boric acid, B(OH) 3 .
-
Suppose First Fidelity Bank engaged in the following transactions: (Click the icon to view the transactions.) Journalize the 2018 and 2019 transactions on First Fidelity's books. Explanations are not...
-
Financial data for Joel de Paris, Inc., for last year follow: Joel de Paris, Inc. Balance Sheet Beginning Balance Ending Balance Assets Cash Accounts receivable Inventory Plant and equipment, net...
-
Supply costs at Coulthard Corporation's chain of gyms are listed below: March April May June July August September October November Client-Visits 11,666 11,462 11,994 13,900 11,726 11, 212 12,006...

Study smarter with the SolutionInn App


