ATP synthase is the worlds smallest rotary motor. Passage of H + ions through the membrane embedded
Question:
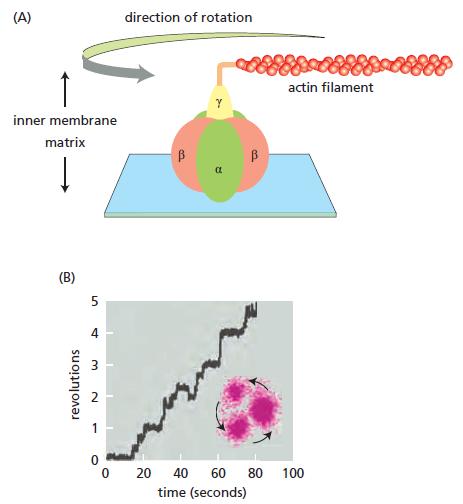
ATP synthase is the world’s smallest rotary motor. Passage of H+ ions through the membrane embedded portion of ATP synthase (the Fo component) causes rotation of the single, central, axle-like γ subunit inside the head group. The tripartite head is composed of the three αβ dimers, the β subunit of which is responsible for synthesis of ATP. The rotation of the γ subunit induces conformational changes in the αβ dimers that allow ADP and Pi to be converted into ATP. A variety of indirect evidence had suggested rotary catalysis by ATP synthase, but seeing is believing.
To demonstrate rotary motion, a modified form of the α3β3γ complex was used. The β subunits were modified so they could be firmly anchored to a solid support and the γ subunit was modified (on the end that normally inserts into the Fo component in the inner membrane) so that a fluorescently tagged, readily visible filament of actin could be attached (Figure Q14–2A). This arrangement allows rotations of the γ subunit to be visualized as revolutions of the long actin filament. In these experiments, ATP synthase was studied in the reverse of its normal mechanism by allowing it to hydrolyze ATP. At low ATP concentrations, the actin filament was observed to revolve in steps of 120° and then pause for variable lengths of time, as shown in Figure Q14–2B.
A. Why does the actin filament revolve in steps with pauses in between? What does this rotation correspond to in terms of the structure of the α3β3γ complex?
B. In its normal mode of operation inside the cell, how many ATP molecules do you suppose would be synthesized for each complete 360° rotation of the γ subunit? Explain your answer.
Figure Q14-2
Step by Step Answer:

Molecular Biology Of The Cell
ISBN: 9780815344322
6th Edition
Authors: Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter




